Label: TIZANIDINE HYDROCHLORIDE capsule
- NDC Code(s): 80425-0474-1, 80425-0474-2, 80425-0474-3
- Packager: Advanced Rx of Tennessee, LLC
- This is a repackaged label.
- Source NDC Code(s): 72888-002
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 20, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONTIZANIDINE HYDROCHLORIDE capsules - These highlights do not include all the information needed to use TIZANIDINE HYDROCHLORIDE CAPSULES safely and effectively. See full prescribing information for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETizanidine hydrochloride is a central alpha-2-adrenergic agonist indicated for the management of spasticity. Because of the short duration of therapeutic effect, treatment with tizanidine ...
-
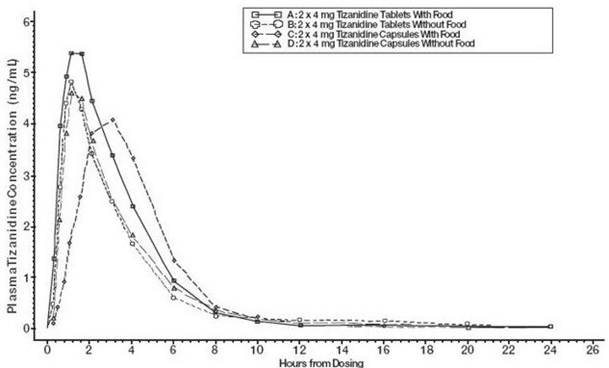
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - Tizanidine hydrochloride capsules may be prescribed with or without food. Once the formulation has been selected and the decision to take with or without food has been ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules - 2 mg: Size 5 Hard gelatin capsule filled with off white to pale yellow granular powder. Cap: Blue opaque, imprinted with " ^" in black ink and Body: Light Blue opaque imprinted with ...
-
4 CONTRAINDICATIONSTizanidine hydrochloride capsules is contraindicated in patients taking potent inhibitors of CYP1A2, such as fluvoxamine or ciprofloxacin - [see Drug Interactions ( 7.1, 7.2) ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension - Tizanidine is an α 2-adrenergic agonist that can produce hypotension. Syncope has been reported in the post marketing setting. The chance of significant hypotension may ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in other sections of the prescribing information: Hypotension - [see Warnings and Precautions ( 5.1)] Liver Injury ...
-
7 DRUG INTERACTIONS7.1 Fluvoxamine - Concomitant use of fluvoxamine and tizanidine is contraindicated. Changes in pharmacokinetics of tizanidine when administered with fluvoxamine resulted in significantly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C - Tizanidine has not been studied in pregnant women. Tizanidine should be given to pregnant women only if the benefit outweighs the risk to the unborn fetus ...
-
9 DRUG ABUSE AND DEPENDENCE9.2 Abuse - Abuse potential was not evaluated in human studies. Rats were able to distinguish tizanidine from saline in a standard discrimination paradigm, after training, but failed to ...
-
10 OVERDOSAGEA review of the safety surveillance database revealed cases of intentional and accidental tizanidine overdose. Some of the cases resulted in fatality and many of the intentional overdoses were ...
-
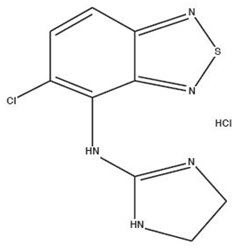
11 DESCRIPTIONTizanidine Hydrochloride is a central alpha2-adrenergic agonist. Tizanidine Hydrochloride is a almost white to slightly yellow, crystalline powder. Tizanidine is slightly soluble in water and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Tizanidine was administered to mice for 78 weeks at oral doses up to 16 mg/kg/day, which is 2 times the maximum ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tizanidine is a central alpha-2-adrenergic receptor agonist and presumably reduces spasticity by increasing presynaptic inhibition of motor neurons. The effects of ...
-
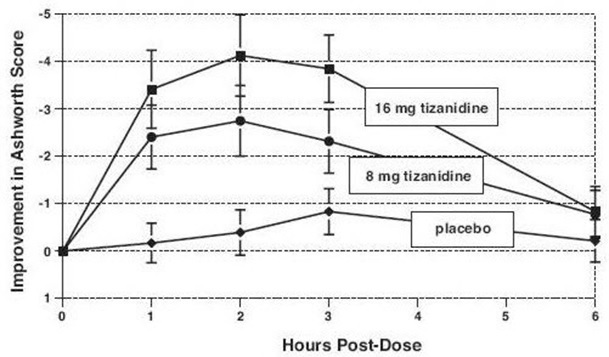
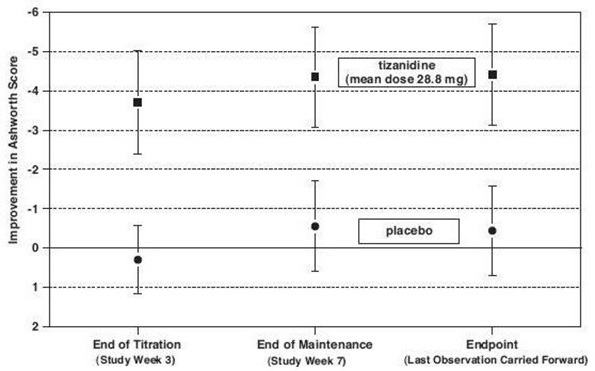
14 CLINICAL STUDIESTizanidine’s capacity to reduce increased muscle tone associated with spasticity was demonstrated in two adequate and well controlled studies in patients with multiple sclerosis or spinal cord ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 Tizanidine Hydrochloride Capsules - Tizanidine Hydrochloride Capsules are available in as two-piece hard gelatin capsules containing tizanidine hydrochloride 4.58 mg equivalent to 4 mg ...
-
17 PATIENT COUNSELING INFORMATIONSerious Drug Interactions - Advise patients they should not take tizanidine if they are taking fluvoxamine or ciprofloxacin because of the increased risk of serious adverse reactions including ...
-
PRINCIPAL DISPLAY PANEL - 4 mg
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information