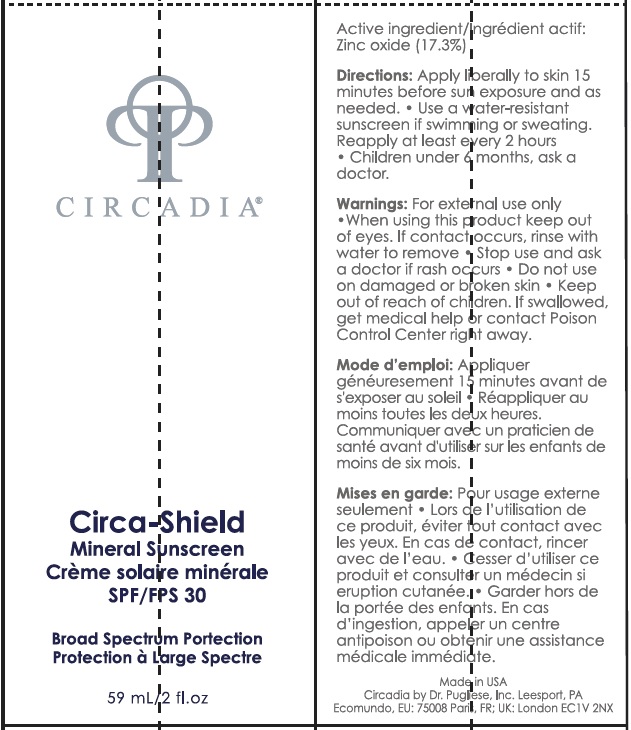
Label: CIRCADIA CIRCA-SHIELD MINERAL SUNSCREEN SPF 30- zinc oxide cream
- NDC Code(s): 76458-401-00
- Packager: Circadia by Dr Pugliese, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
- Warnings
-
Directions
apply liberally to skin 15 minutes before sun exposure.
- use a water resistant sunscreen if swimming or sweating.
- reapply at least every 2 hours.
- children under 6 months: Ask a doctor.
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with Broad Spectrum SPF or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirt, pants, hats and sunglasses.
-
Inactive Ingredients
C14-22 Alkane, Olea Europaea fruit Oil, Sorbitan Olivate, Diisopropyl Adipate, C9-12 Alkane, Silica, Oryza Sativa Bran Cera, Vegetable Oil, Simmondsia Chinensis Seed Oil, Curcuma Longa Root Extract, Saccharomyces Ferment, Polyglyceryl-4 Olivate/Polyricinoleate, Triethoxycaprylylsilane, Coco-Caprylate, Glycine Soja Oil, Polyhydroxystearic Acid, Triethyl Citrate, Lauroyl Lysine, Bis(Cyano Butylacetate) Antharacenediylidene, Polyglyceryl-3 Diisostearate, Oryza Sativa Germ Extract Oryza Sativa Extract, Caprylic /Capric Triglyceride, Camellia Sinensis Leaf Extract, Vanila Planifolia Fruit Extract, Salvia Officinalis Flower/Leaf/Stem Extract, Rosmarinus Officinalis Leaf Extract, Lavandula Angustifolia Flower/Leaf/Stem Extract, Salvia Triloba Leaf Extract, Jasminum Officinale Flower/Leaf Extract, Rosa Damascena Flower Extract, Linalool.
- Other information
- Questions?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
CIRCADIA CIRCA-SHIELD MINERAL SUNSCREEN SPF 30
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76458-401 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 173 mg in 1 mL Inactive Ingredients Ingredient Name Strength OLIVE OIL (UNII: 6UYK2W1W1E) SORBITAN OLIVATE (UNII: MDL271E3GR) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) C9-12 ALKANE (UNII: 7J5R5W72QM) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) JOJOBA OIL (UNII: 724GKU717M) TURMERIC (UNII: 856YO1Z64F) POLYGLYCERYL-4 OLIVATE/POLYRICINOLEATE (UNII: WYX2KZL5BD) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) COCO-CAPRYLATE (UNII: 4828G836N6) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) LAUROYL LYSINE (UNII: 113171Q70B) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) VANILLA BEAN (UNII: Q74T35078H) ROSEMARY (UNII: IJ67X351P9) LAVANDULA ANGUSTIFOLIA SUBSP. ANGUSTIFOLIA FLOWER (UNII: 19AH1RAF4M) THREE-LOBE SAGE (UNII: 3V97D33N0K) JASMINUM OFFICINALE FLOWER (UNII: 0Q8K841432) ROSA X DAMASCENA FLOWER (UNII: JWB78P295A) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76458-401-00 1 in 1 BOX 12/31/2023 1 59 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/31/2023 Labeler - Circadia by Dr Pugliese, Inc. (013694423)