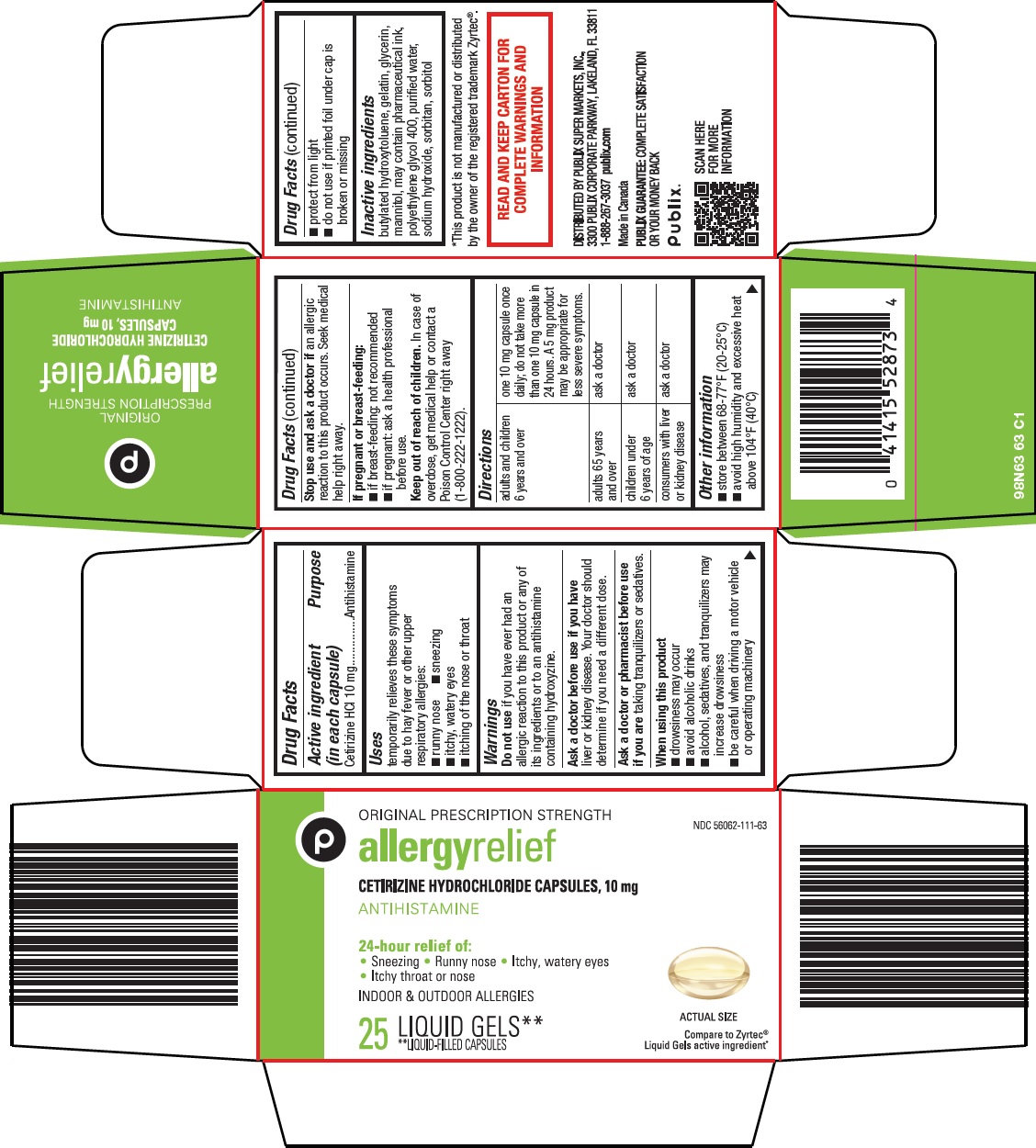
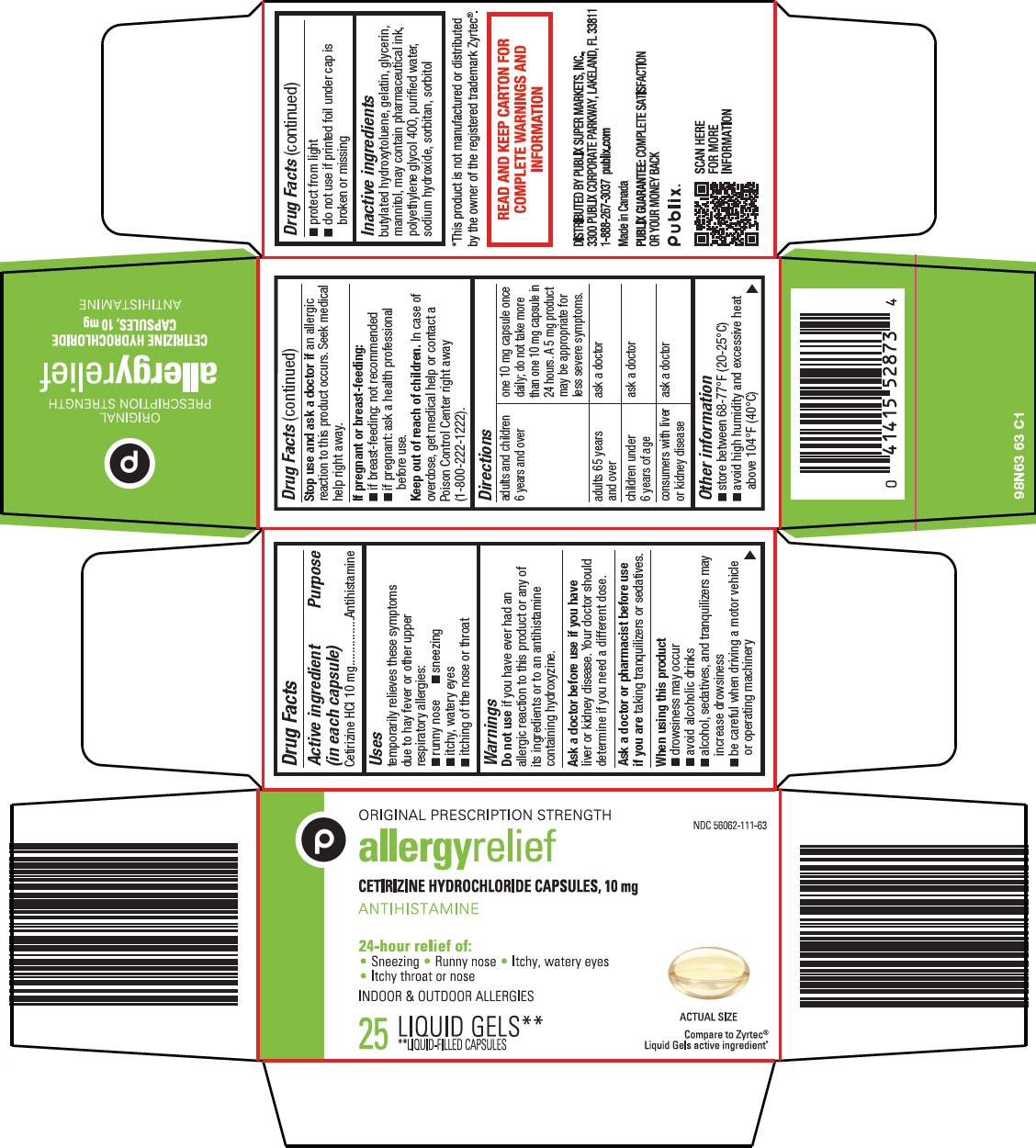
Label: ALLERGY RELIEF- cetirizine hydrochloride capsule, liquid filled
- NDC Code(s): 56062-111-63
- Packager: Publix Super Markets Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 7, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each capsule) Cetirizine HCl 10 mg
-
Purpose Antihistamine
-
Uses temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: • runny nose - • sneezing - • itchy, watery eyes - • itching of the nose or throat
-
Warnings Do not use - if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine. Ask a doctor before use if you have - liver or ...
-
Directions adults and children 6 years and over - one 10 mg capsule once daily; do not take more than one 10 mg capsule in 24 hours. A 5 mg product may be appropriate for less severe ...
-
Other information • store between 68-77°F (20-25°C) • avoid high humidity and excessive heat above 104°F (40°C) • protect from light - • do not use if printed foil under cap is broken or missing
-
Inactive ingredients butylated hydroxytoluene, gelatin, glycerin, mannitol, may contain pharmaceutical ink, polyethylene glycol 400, purified water, sodium hydroxide, sorbitan, sorbitol
-
Package/Label Principal Display Panel ORIGINAL PRESCRIPTION STRENGTH - allergy relief - CETIRIZINE HYDROCHLORIDE CAPSULES, 10 mg - ANTIHISTAMINE - 24-hour relief of: • Sneezing • Runny nose • Itchy, watery eyes • Itchy throat or nose - INDOOR ...
-
INGREDIENTS AND APPEARANCEProduct Information