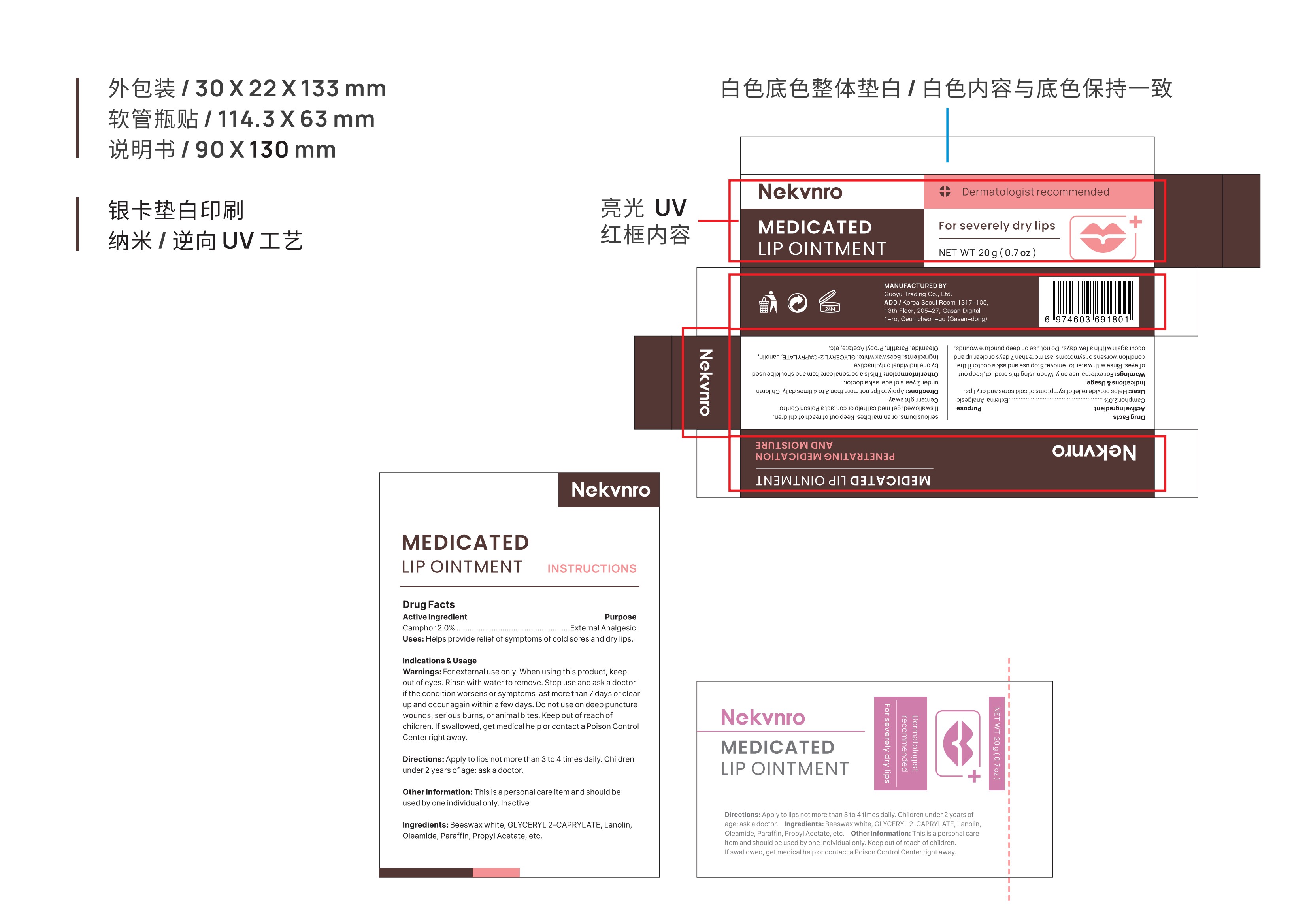
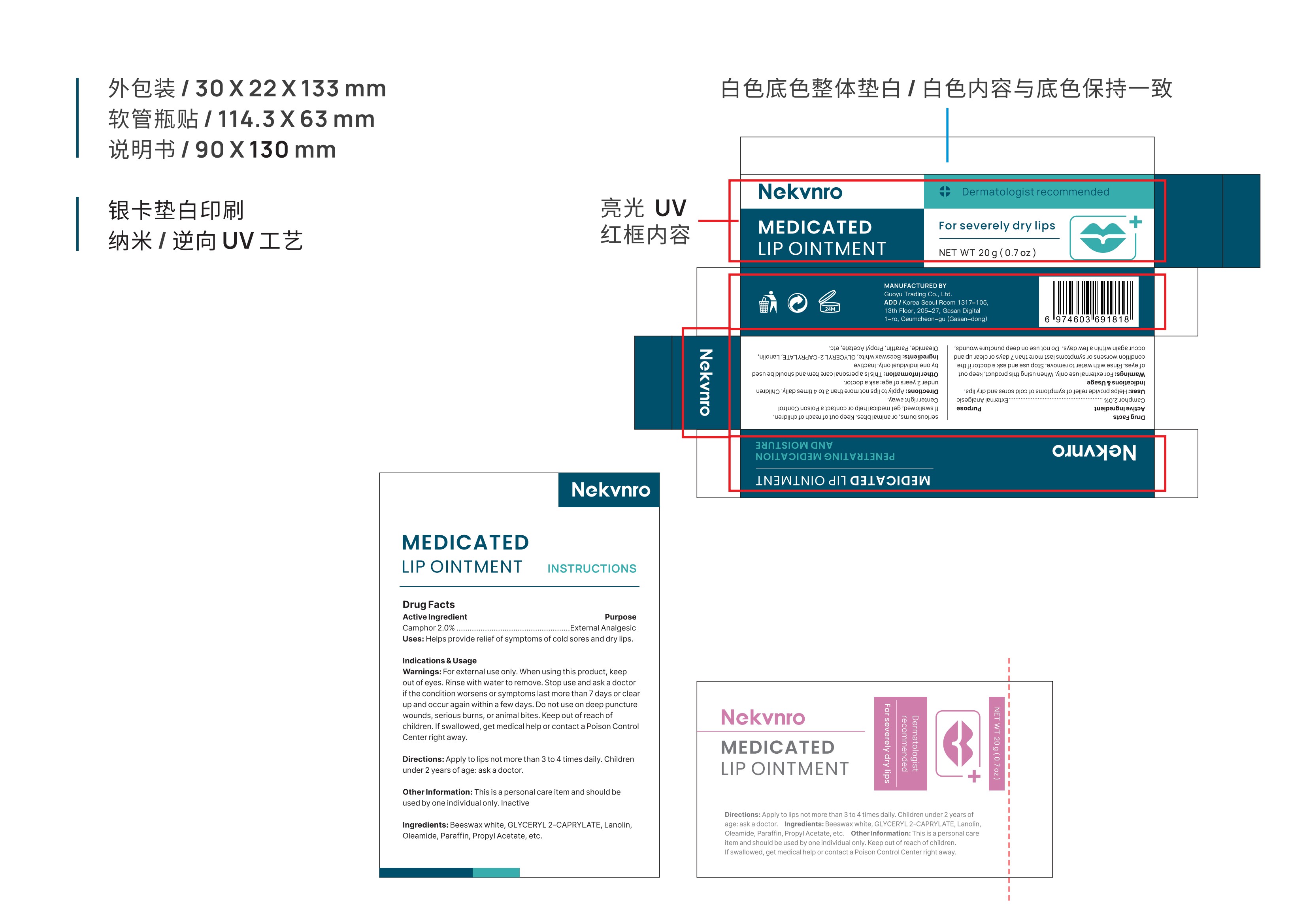
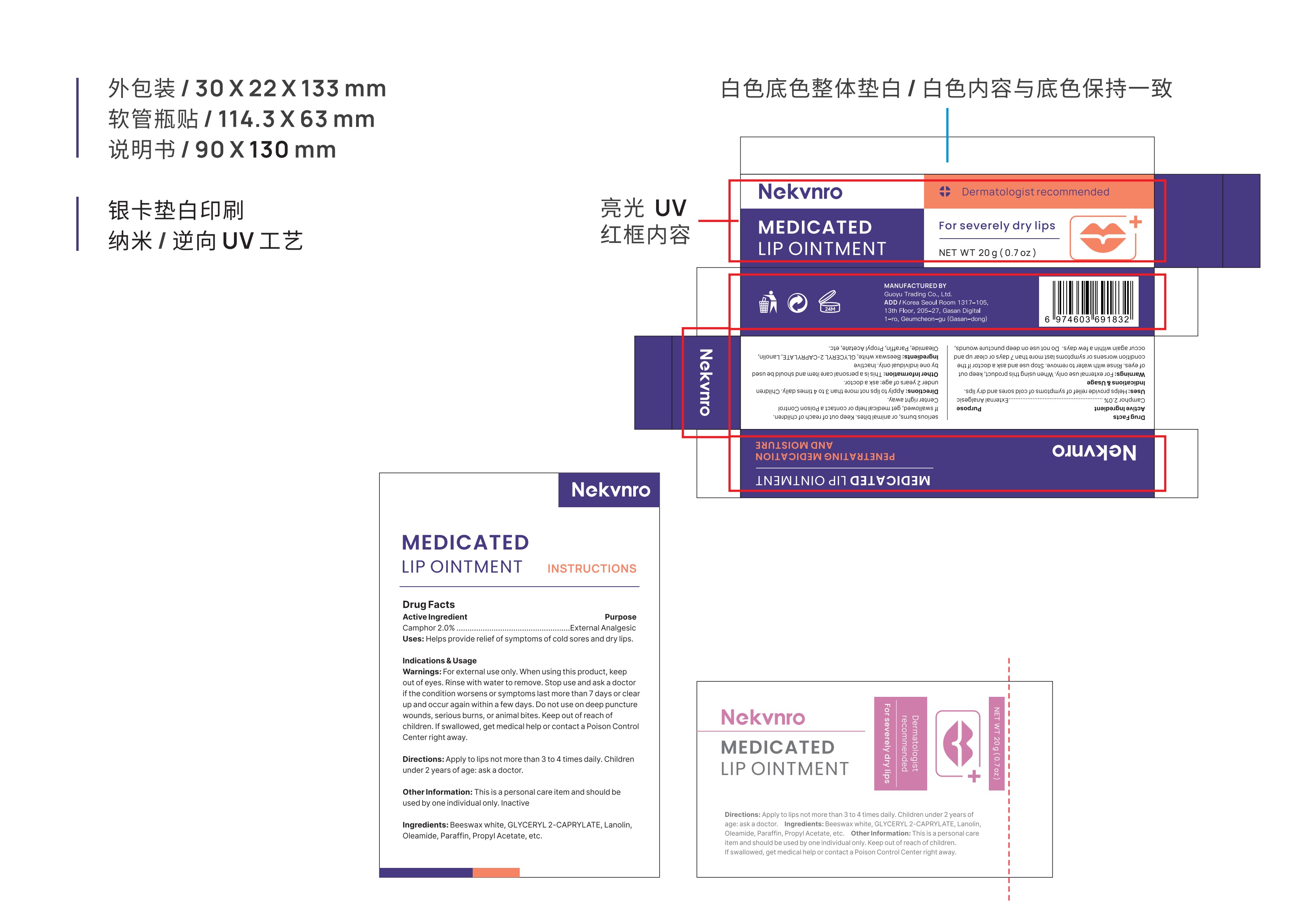
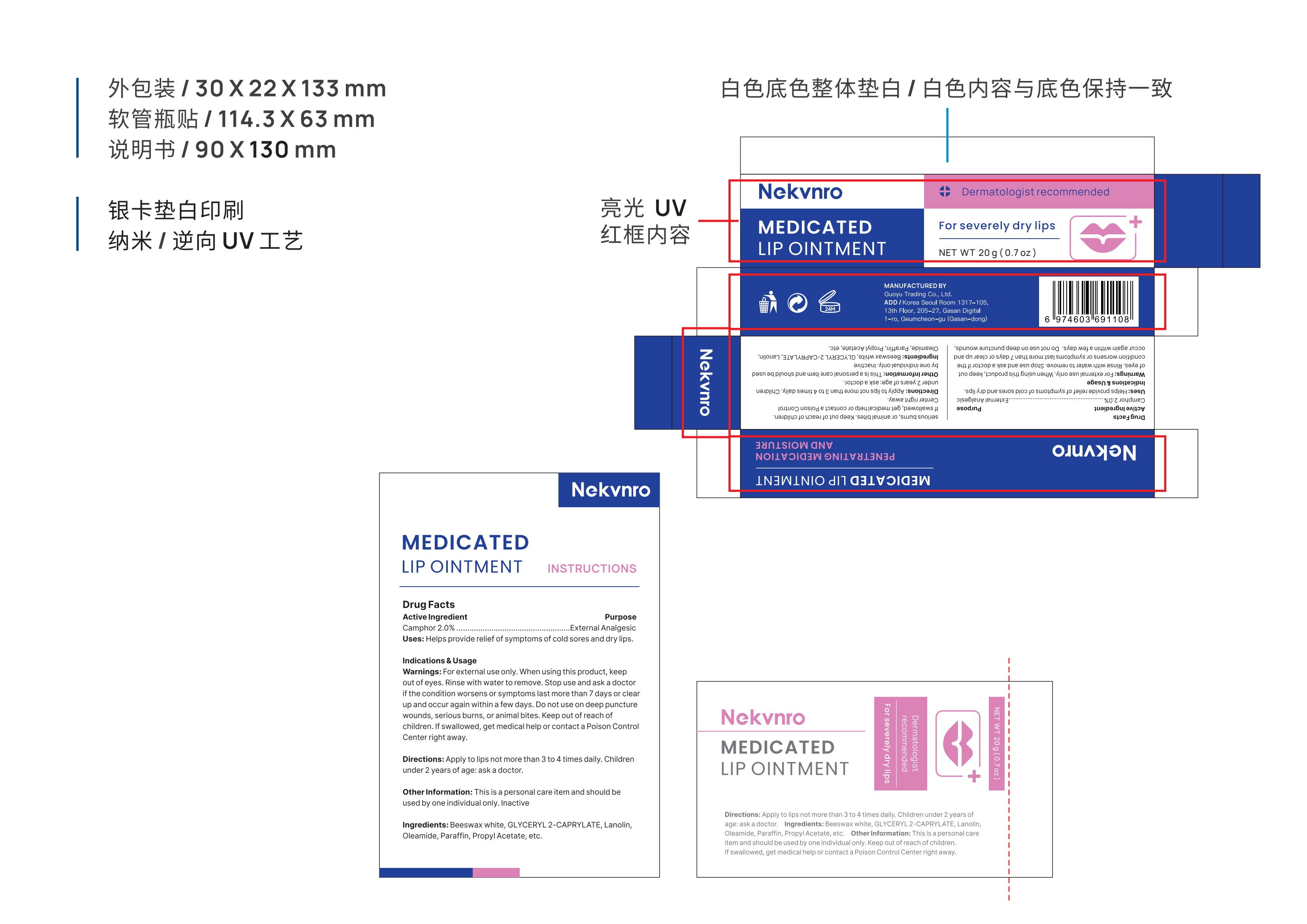
Label: NEKVNRO MEDICATED LIP OINTMENT.- medicated lip ointment ointment
- NDC Code(s): 84844-004-01, 84844-004-02
- Packager: Guoyu Trading Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEKVNRO MEDICATED LIP OINTMENT.
medicated lip ointment ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84844-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 2 g in 100 g Inactive Ingredients Ingredient Name Strength LANOLIN (UNII: 7EV65EAW6H) PROPYL ACETATE (UNII: 4AWM8C91G6) PARAFFIN (UNII: I9O0E3H2ZE) WHITE WAX (UNII: 7G1J5DA97F) GLYCERYL 2-CAPRYLATE (UNII: O8TCW8DUW8) OLEAMIDE (UNII: 7L25QK8BWO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84844-004-01 10 g in 1 TUBE; Type 0: Not a Combination Product 10/31/2024 2 NDC:84844-004-02 20 g in 1 TUBE; Type 0: Not a Combination Product 10/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/31/2024 Labeler - Guoyu Trading Co., Ltd. (963348600) Establishment Name Address ID/FEI Business Operations Guoyu Trading Co., Ltd. 963348600 manufacture(84844-004)