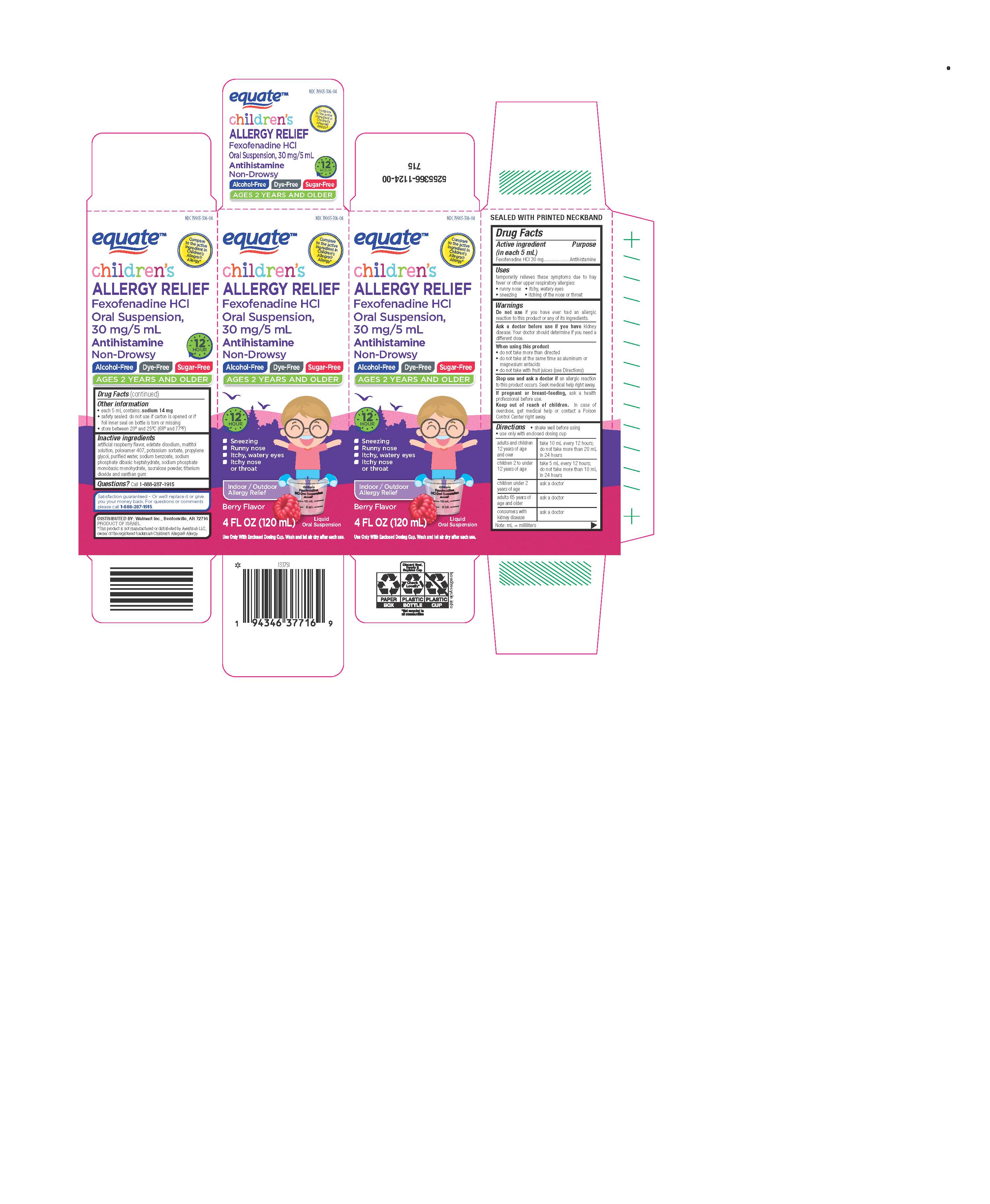
Label: CHILDRENS FEXOFENADINE HYDROCHLORIDE ALLERGY- fexofenadine hydrochloride suspension
- NDC Code(s): 79903-306-04
- Packager: Wal-Mart Stores Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredient (in each 5 mL)Fexofenadine HCl 30 mg
-
PurposeAntihistamine
-
Usestemporarily relieves these symptoms due to hay fever or other upper respiratory allergies: runny nose - itchy, watery eyes - sneezing - itching of the nose or throat
-
WarningsDo not use if you have ever had an allergic reaction to this product or any of its ingredients. Ask a doctor before use if you have kidney disease. Your doctor should determine if you ...
-
Directionsshake well before using - use only with enclosed dosing cup - Note: mL = milliliters - adults and children 12 years of age and overtake 10 mL every 12 hours; do not take more than 20 mL in ...
-
Other informationeach 5 mL contains: sodium 14 mg - safety sealed: do not use if carton is opened, or if foil inner seal on bottle is torn or missing - store between 20º and 25ºC (68º and 77ºF)
-
Inactive ingredientsartificial raspberry flavor, edetate disodium, maltitol solution, poloxamer 407, potassium sorbate, propylene glycol, purified water, sodium benzoate, sodium phosphate dibasic heptahydrate, sodium ...
-
Questions?Call - 1-888-287-1915
-
SPL UNCLASSIFIED SECTIONDISTRIBUTED BY: Walmart Inc., Bentonville, AR 72716
-
PRINCIPAL DISPLAY PANEL - 120 mL Bottle CartonNDC 79903-306-04 - Compare to the active - ingredient in Children's - Allegra - ®Allergy* Children's - Allergy Relief - Fexofenadine HCl - Oral Suspension, 30 mg/ 5 mL ...
-
INGREDIENTS AND APPEARANCEProduct Information