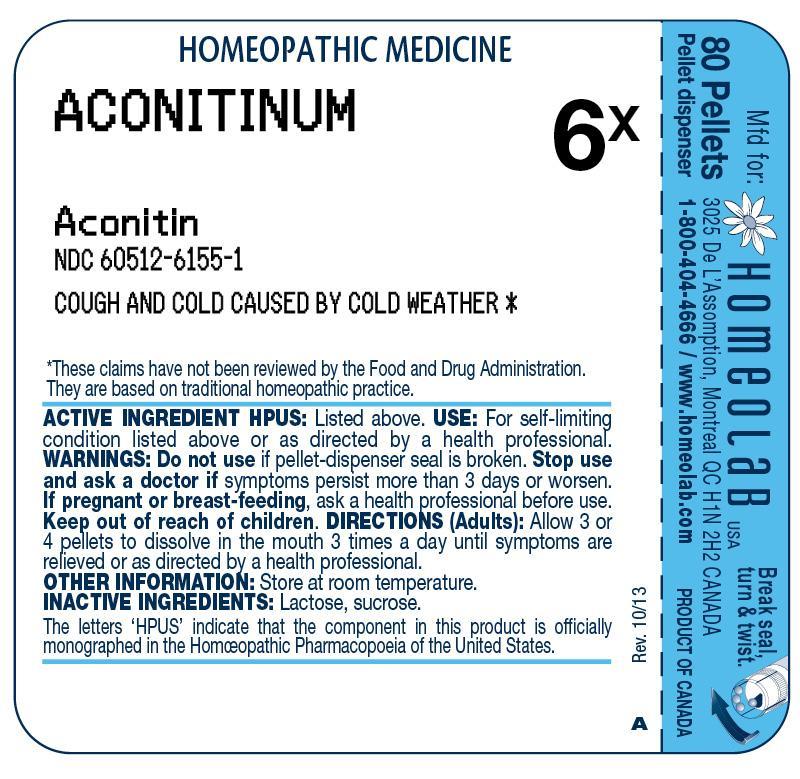
Label: ACONITINUM pellet
-
Contains inactivated NDC Code(s)
NDC Code(s): 60512-6155-1 - Packager: HOMEOLAB USA INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 17, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
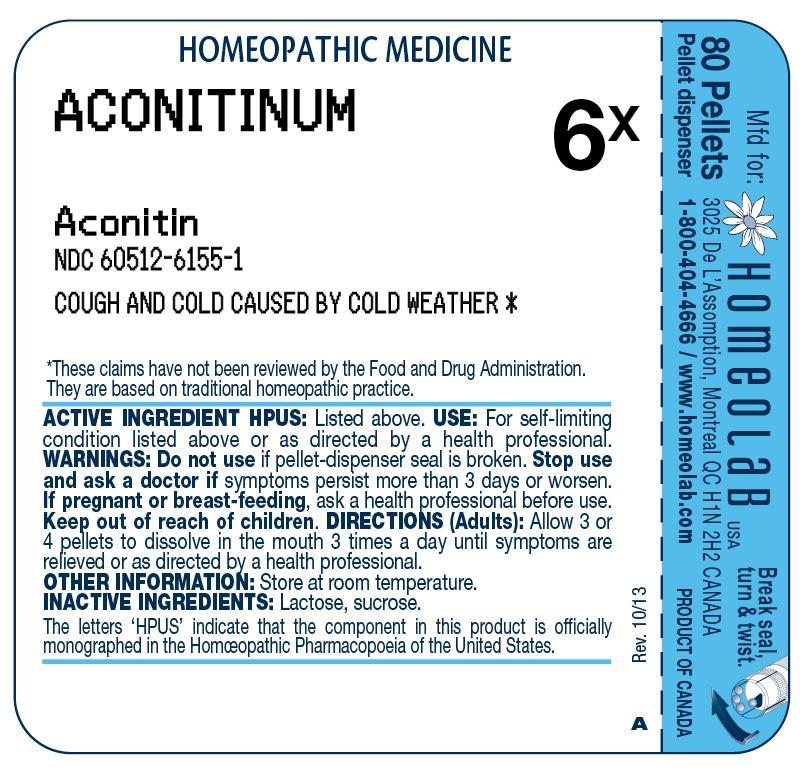
ACTIVE INGREDIENT HPUSACONITINUM 6X - (Aconitin)
-
PURPOSECOUGH & COLD CAUSED BY COLD WEATHER
-
USEFor self-limiting condition listed above or as directed by a health professional.
-
WARNINGSDo not use if pellet-dispenser seal is broken. Stop use and ask a doctor if symptoms persist more than 3 days or worsen. If pregnant or breast-feeding, ask a health professional ...
-
DIRECTIONSAdults: Allow 3 or 4 pellets to dissolve in the mouth 3 times a day until symptoms are relieved or as directed by a health professional.
-
OTHER INFORMATIONStore at room temperature.
-
INACTIVE INGREDIENTSLactose, sucrose.
-
QUESTIONS?1-800-404-4666
-
REFERENCESThe letters 'HPUS' indicate that the component in this product is officially monographed in the Homeopathic Pharmacopoeia of the United States. These claims have not been reviewed by the Food ...
-
DESCRIPTION80 Pellets - Pellet dispenser - Mfd for: HOMEOLAB USA INC., 3025 De L`Assomption, Montreal, QC, H1N 2H2, CANADA - Product of Canada
-
PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information