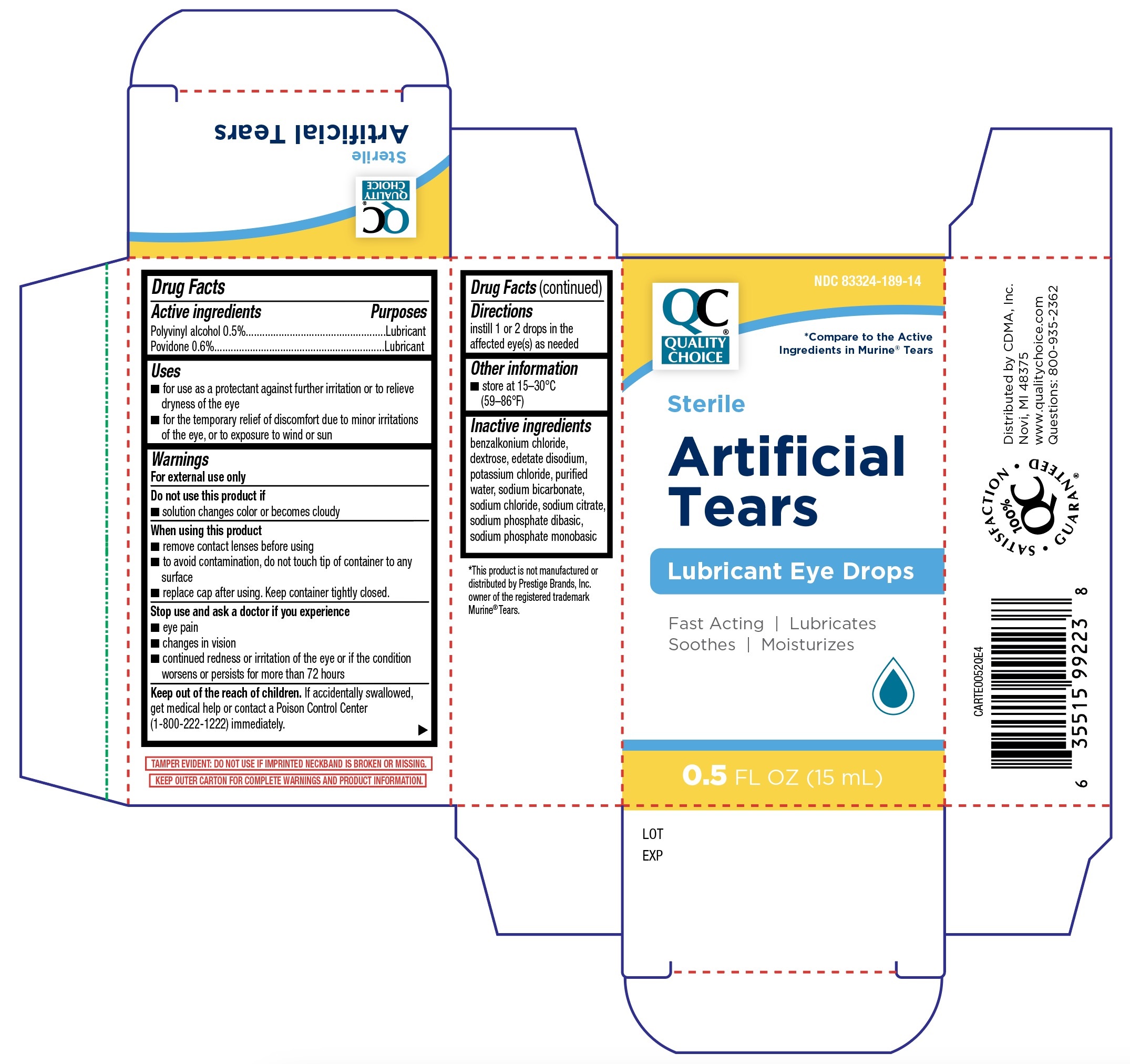
Label: QUALITY CHOICE ARTIFICIAL TEARS LUBRICANT EYE DROPS- polyvinyl alcohol, povidone solution/ drops
- NDC Code(s): 83324-189-14
- Packager: Chain Drug Marketing Association, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active IngredientsPolyvinyl alcohol 0.5% Povidone 0.6%
-
PurposeLubricant - Lubricant
-
Usesfor use as a protectant against further irritation or to relieve dryness of the eye - for the temporary relief of discomfort due to minor irritations of the eye, or to exposure to wind or ...
-
WarningsFor external use only - Do not use this product if - solution changes color or becomes cloudy - When using this product - remove contact lens before using - to avoid contamination, do not ...
-
DirectionsInstill 1 or 2 drops in the affected eye(s) as needed.
-
Other information
Store at 15°-30°C (59°-86°F)
-
Inactive ingredients
benzalkonium chloride, dextrose, edetate disodium, potassium chloride, purified water, sodium bicarbonate, sodium chloride, sodium citrate, sodium phosphate dibasic, and sodium phosphate ...
-
Quality Choice Artificial Tears Lubricant Eye Drops 15mL
-
INGREDIENTS AND APPEARANCEProduct Information

