Label: zolpidem tartrate- Zolpidem tartrate tablet for oral use - CIV
-
Contains inactivated NDC Code(s)
NDC Code(s): 68462-279-01, 68462-279-05, 68462-280-01, 68462-280-05 - Packager: InvaGen Pharmaceuticals Inc.,
- Category: HUMAN PRESCRIPTION DRUG LABEL WITH HIGHLIGHTS
- DEA Schedule: CIV
Drug Label Information
Updated February 11, 2008
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONRECENT MAJOR CHANGES - Indications and Usage (1) 03/2007 - Warnings and Precautions ...
-
Table of ContentsTable of Contents
- N/A - Section Title Not Found In Database
-
1 INDICATIONS AND USAGEZolpidem tartrate tablets are indicated for the short-term treatment of insomnia characterized by difficulties with sleep initiation. Zolpidem tartrate tablets have been shown to ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Dosage in adults - The dose of zolpidem tartrate tablets should be individualized. The recommended dose for adults is 10 mg immediately before bedtime. Zolpidem tartrate tablets should not be ...
-
3 DOSAGE FORMS AND STRENGTHSZolpidem tartrate tablets are available in 5 mg and 10 mg strength tablets for oral administration. Zolpidem tartrate 5 mg tablets are pink, film coated, modified oval shaped, biconvex tablets ...
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - Zolpidem tartrate tablets are contraindicated in patients with known hypersensitivity to zolpidem tartrate or to any of the inactive ingredients in the formulation. (Also ...
-
5 WARNINGS AND PRECAUTIONS5.1 General - Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful ...
-
6 ADVERSE REACTIONSSerious adverse reactions including severe anaphylactic and anaphylactoid reactions, abnormal thinking and behavior, complex behaviors, withdrawal effects, amnesia, anxiety, other ...
-
7 DRUG INTERACTIONS7.1 CNS-active drugs - Since the systematic evaluations of zolpidem tartrate tablets in combination with other CNS-active drugs have been limited, careful consideration should be given to the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic effects: Pregnancy Category C - Zolpidem tartrate was administered to pregnant Sprague-Dawley rats by oral gavage during the period of organogenesis at doses of 4 ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 CONTROLLED SUBSTANCE - Zolpidem tartrate is classified as a Schedule IV controlled substance by federal regulation. 9.2 ABUSE - Abuse and addiction are separate and distinct from physical ...
-
10 OVERDOSAGE10.1 Signs and symptoms - In postmarketing experience of overdose with zolpidem alone, or in combination with CNS-depressant agents, impairment of consciousness ranging from somnolence to coma ...
-
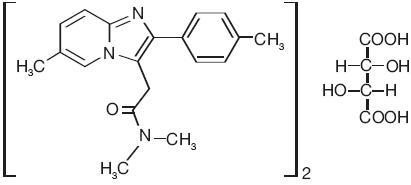
11 DESCRIPTIONZolpidem tartrate is a non-benzodiazepine hypnotic of the imidazopyridine class and is available in 5 mg and 10 mg strength tablets for oral administration. Chemically, zolpidem is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Subunit modulation of the GABAA receptor chloride channel macromolecular complex is hypothesized to be responsible for sedative, anticonvulsant, anxiolytic, and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: Zolpidem was administered to rats and mice for 2 years at dietary dosages of 4, 18, and 80 mg/kg/day. In mice, these ...
-
14 CLINICAL STUDIES14.1 Transient insomnia - Normal adults experiencing transient insomnia (n = 462) during the first night in a sleep laboratory were evaluated in a double-blind, parallel group, single-night trial ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Zolpidem tartrate tablets, 5-mg are pink, film coated, modified oval shaped, biconvex tablets debossed with IG on one side and 259 on other and supplied as: NDC ...
-
17 PATIENT COUNSELING INFORMATIONSee Medication Guide. Prescribers or other healthcare professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with ...
-
INGREDIENTS AND APPEARANCEProduct Information