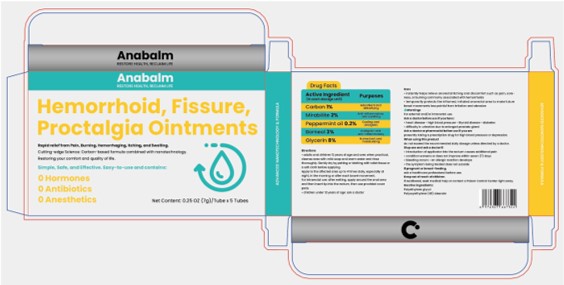
Label: HEMORRHOID, FISSURE, PROCTALGIA OINTMENTS- carbon, mirabilite, peppermint oil, borneol, glycerin salve
- NDC Code(s): 84533-003-01
- Packager: WU XI ZHONG ZHI WEI NA TECHNOLOGY CO., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
.aduits and chlkdren 12 years of oage and oer when practical,cleanse area with mild soap and warm water and rinsethorcughiy, Gently dry by patting or blotting with tollet tissue ora sodt cloth before applying.Apply to the atfected area up to 4 times daily, cspecially atnight, in the moming or after each bowei movement.For intrarectaluse: after watting, apply around the anad creaand then insert tip into tho reatum, then use provided oowerpads.
. children under 12 yoars cf age ask a doctor - INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMORRHOID, FISSURE, PROCTALGIA OINTMENTS
carbon, mirabilite, peppermint oil, borneol, glycerin salveProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84533-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 2 g in 100 g BORNEOL (UNII: M89NIB437X) (BORNEOL - UNII:M89NIB437X) BORNEOL 3 g in 100 g ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 1 g in 100 g PEPPERMINT OIL (UNII: AV092KU4JH) (PEPPERMINT - UNII:V95R5KMY2B) PEPPERMINT OIL 0.2 g in 100 g GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 8 g in 100 g Inactive Ingredients Ingredient Name Strength PEG-23 STEARATE (UNII: RCE8XV9D25) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84533-003-01 7 g in 1 BOX; Type 0: Not a Combination Product 08/06/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 08/06/2024 Labeler - WU XI ZHONG ZHI WEI NA TECHNOLOGY CO., Ltd. (412779339) Establishment Name Address ID/FEI Business Operations WU XI ZHONG ZHI WEI NA TECHNOLOGY CO., Ltd. 412779339 manufacture(84533-003)