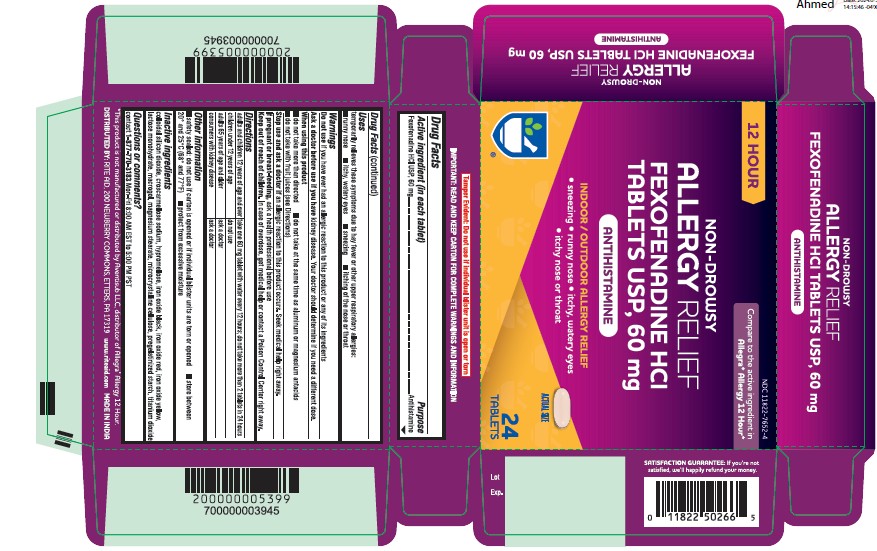
Label: FEXOFENADINE HCL tablet
- NDC Code(s): 11822-7652-4
- Packager: Rite Aid
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each tablet)
Fexofenadine HCl USP, 60 mg
-
Purpose
Antihistamine
-
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: ■ runny nose ■ itchy, watery eyes ■ sneezing ■ itching of the nose or throat
-
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients
-
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
-
When using this product
■ do not take more than directed ■ do not take at the same time as aluminum or magnesium antacids - ■ do not take with fruit juices (see Directions)
-
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
-
If pregnant or breast-feeding,
ask a health professional before use
-
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
-
Directions
adults and children take one 60 mg tablet with water every 12 hours; do not take more than 2 tablets in 24 hours - 12 years of age and over - children under 12 years ...
-
Other information
■ safety sealed: do not use if carton is opened or if individual blister units are torn or opened ■ store between - 20° and 25°C (68° and 77°F) ■ protect from excessive moisture
-
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, lactose monohydrate, macrogol, magnesium stearate, microcrystalline ...
-
Questions or comments?
contact - 1-877-770-3183 Mon-Fri 8:00 AM EST to 5:00 PM PST
-
PDP
-
INGREDIENTS AND APPEARANCEProduct Information

