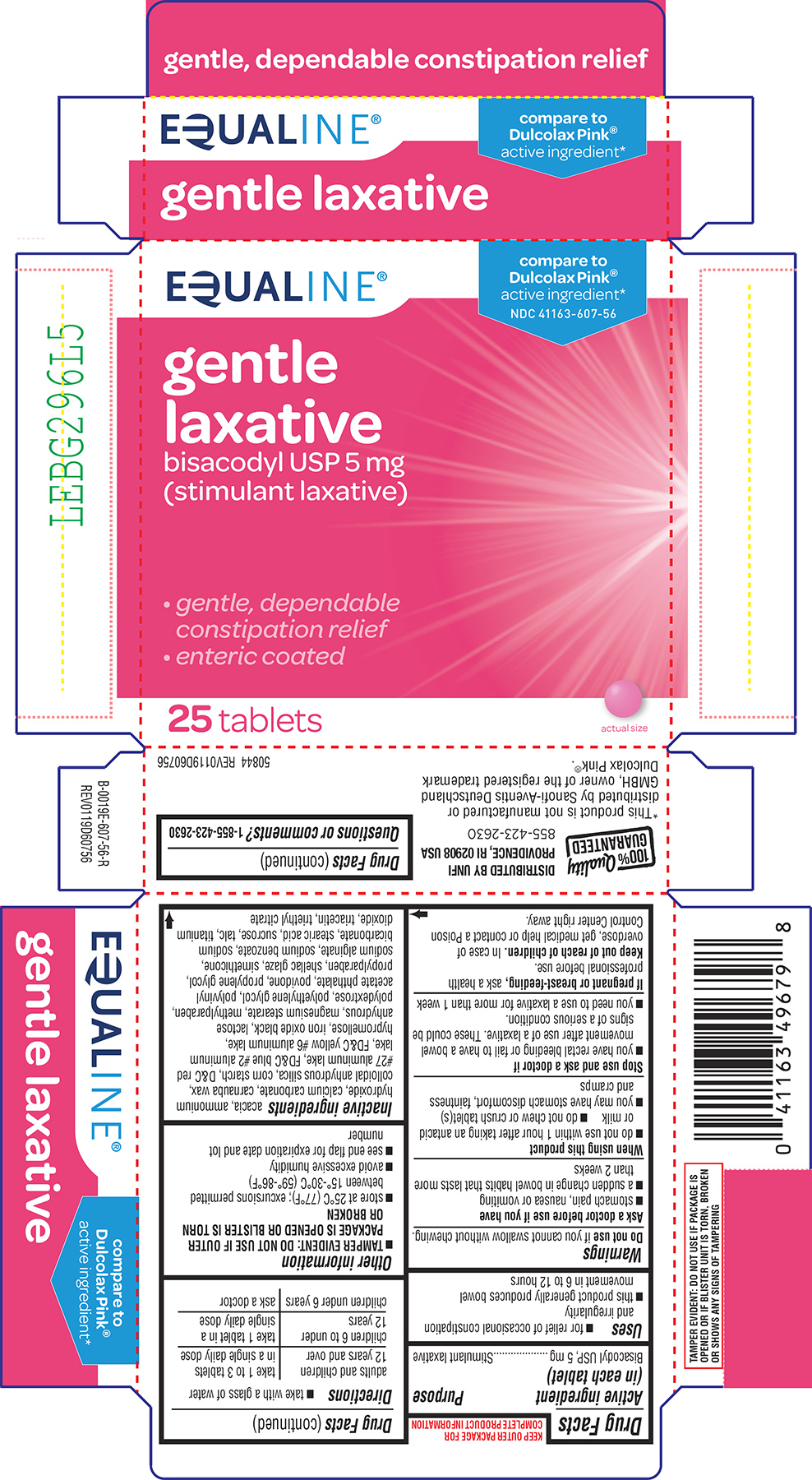
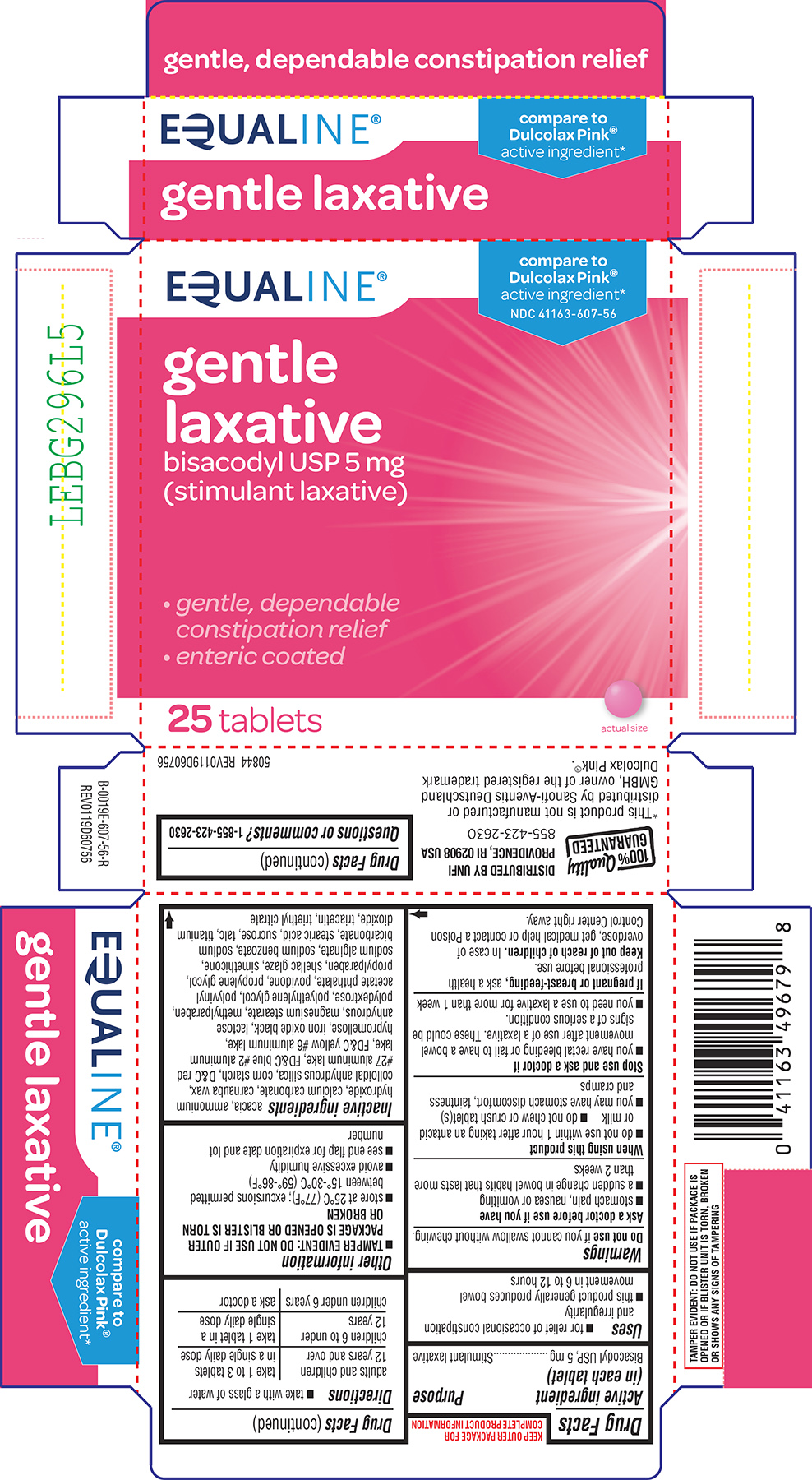
Label: GENTLE LAXATIVE- bisacodyl tablet, delayed release
- NDC Code(s): 41163-607-52, 41163-607-56
- Packager: United Natural Foods, Inc. dba UNFI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
When using this product
- do not chew or crush tablet(s)
- do not use within 1 hour after taking an antacid or milk
- it may cause stomach discomfort, faintness, and cramps
- Directions
- Other information
-
Inactive ingredients
acacia, ammonium hydroxide, calcium carbonate, carnauba wax, colloidal anhydrous silica, corn starch, D&C red #27 aluminum lake, FD&C blue #2 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, iron oxide black, lactose anhydrous, magnesium stearate, methylparaben, polydextrose, polyethylene glycol, polyvinyl acetate phthalate, povidone, propylene glycol, propylparaben, shellac glaze, simethicone, sodium alginate, sodium benzoate, sodium bicarbonate, stearic acid, sucrose, talc, titanium dioxide, triacetin, triethyl citrate
- Questions or comments?
-
Principal Display Panel
EQUALINE®
compare to
Dulcolax Pink®
active ingredient*NDC 41163-607-56
gentle
laxative
bisacodyl USP 5 mg
(stimulant laxative)• gentle, dependable
constipation relief
• enteric coated25 tablets
actual size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING100% Quality
GUARANTEEDDISTRIBUTED BY UNFI
PROVIDENCE, RI 02908 USA
855-423-2630*This product is not manufactured or distributed by A. Nattermann & Cie. GmbH, owner of the
registered trademark Dulcolax Pink®.50844 REV0823E60756
Equaline 44-607
-
INGREDIENTS AND APPEARANCE
GENTLE LAXATIVE
bisacodyl tablet, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41163-607 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISACODYL (UNII: 10X0709Y6I) (DEACETYLBISACODYL - UNII:R09078E41Y) BISACODYL 5 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) AMMONIA (UNII: 5138Q19F1X) CALCIUM CARBONATE (UNII: H0G9379FGK) CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) FD&C BLUE NO. 2 ALUMINUM LAKE (UNII: 4AQJ3LG584) FD&C YELLOW NO. 6 ALUMINUM LAKE (UNII: GYP6Z2JR6Q) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) METHYLPARABEN (UNII: A2I8C7HI9T) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Polyvinyl Acetate Phthalate (UNII: 58QVG85GW3) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SHELLAC (UNII: 46N107B71O) DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) SODIUM ALGINATE (UNII: C269C4G2ZQ) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM BICARBONATE (UNII: 8MDF5V39QO) STEARIC ACID (UNII: 4ELV7Z65AP) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color pink Score no score Shape ROUND Size 6mm Flavor Imprint Code 5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41163-607-56 1 in 1 CARTON 04/01/2013 1 25 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:41163-607-52 5 in 1 CARTON 04/01/2013 2 18 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 04/01/2013 Labeler - United Natural Foods, Inc. dba UNFI (943556183) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(41163-607) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(41163-607) , pack(41163-607) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(41163-607) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(41163-607) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(41163-607)