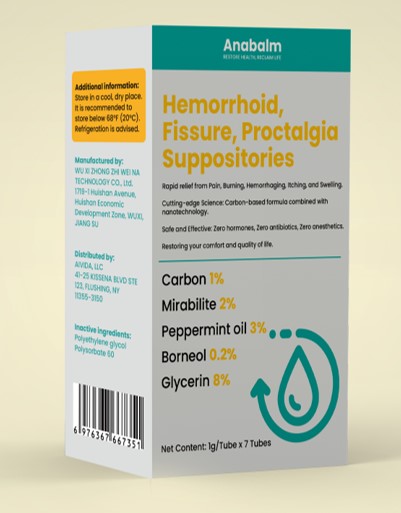
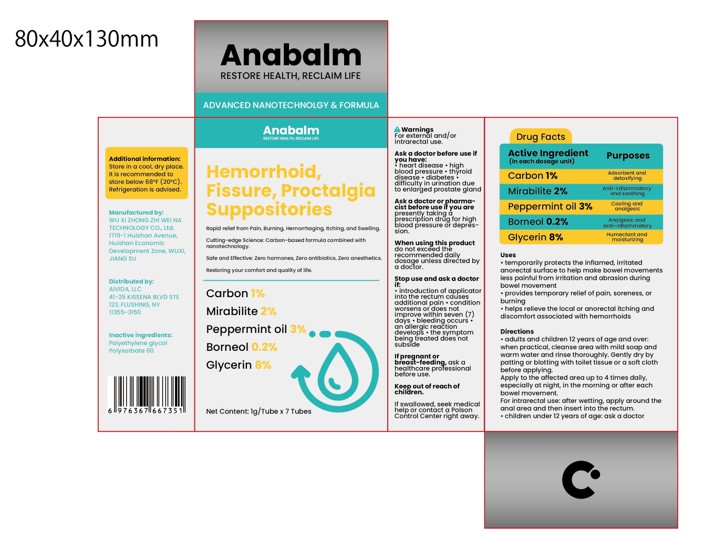
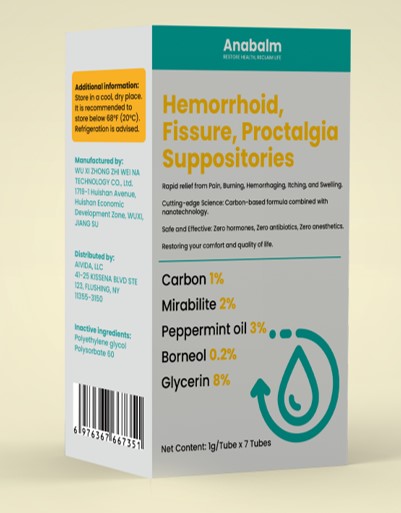
Label: HEMORRHOID, FISSURE, PROCTALGIASUPPOSITORIES- carbon, mirabilite, peppermint oil, borneol suppository
- NDC Code(s): 84533-001-01
- Packager: WU XI ZHONG ZHI WEI NA TECHNOLOGY CO., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
adults and children 12 years of age and over:when practical, cleanse area with mild soap andwarm water and rinse thoroughly.Gently dry bypatting or blotting with toilet tissue or a soft clothbefore applying.Apply to the affected area up to 4 times daily,especially at night, in the morning or after eachbowel movement.For intrarectal use: after wetting, apply around theanal area and then insert into the rectum..children under 12 years of age: ask a doctor
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMORRHOID, FISSURE, PROCTALGIASUPPOSITORIES
carbon, mirabilite, peppermint oil, borneol suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84533-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PEPPERMINT OIL (UNII: AV092KU4JH) (PEPPERMINT - UNII:V95R5KMY2B) PEPPERMINT OIL 0.2 g in 100 g GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 8 g in 100 g ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 1 g in 100 g SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 2 g in 100 g BORNEOL (UNII: M89NIB437X) (BORNEOL - UNII:M89NIB437X) BORNEOL 3 g in 100 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 60 (UNII: CAL22UVI4M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84533-001-01 7 g in 1 BOX; Type 0: Not a Combination Product 07/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 07/17/2024 Labeler - WU XI ZHONG ZHI WEI NA TECHNOLOGY CO., Ltd. (412779339) Establishment Name Address ID/FEI Business Operations WU XI ZHONG ZHI WEI NA TECHNOLOGY CO., Ltd. 412779339 manufacture(84533-001)