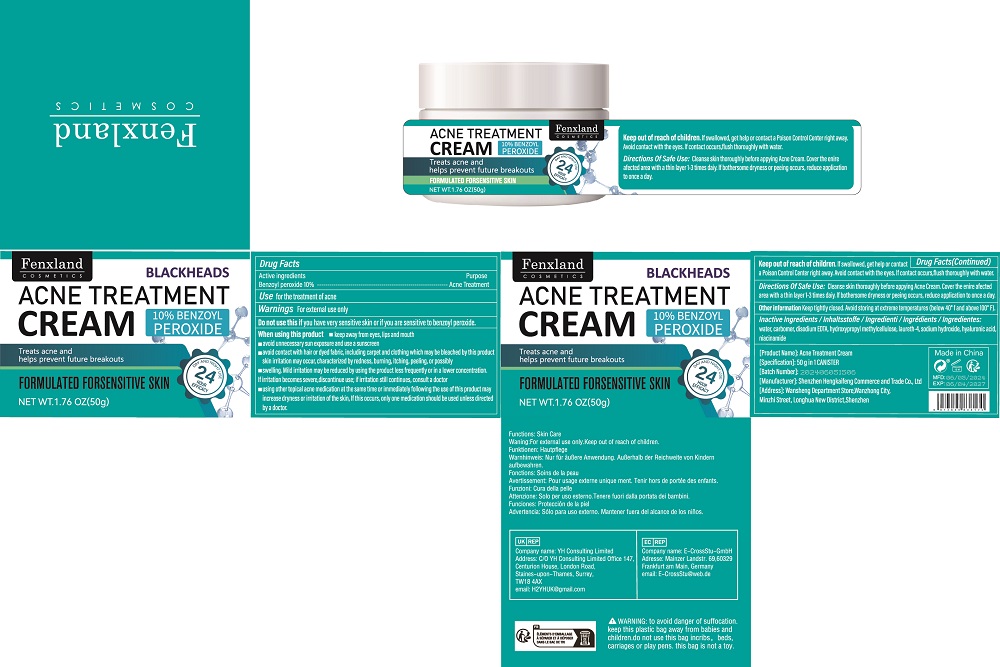
Label: ACNE TREATMENT CREAM cream
- NDC Code(s): 84117-012-01
- Packager: Shenzhen Hengkaifeng Commerce and Trade Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- Use
- Warnings
- Do not use
-
When using this product
keep away from eyes, lips and mouth
wavoid unnecessary sun exposure and use a sunscreen
avoid contact with hair or dyed fabric, including carpet and clothing which may be bleached by this product
skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly
swelling. Mild irritation may be reduced by using the product less frequenty or in a lower concentration.
Ifirritation becomes severe,discontinue use; ifirritation still continues, consulta doctor
wusing other topical acne medication at the same time or immediately following the use of this product may
increase dryness or iritation of the skin, lf this occurs, only one medication should be used unless directed
by a doctor. - Keep Out Of Reach Of Children
- Directions Of Safe Use:
- Other information
- Inactive ingredients
-
DOSAGE & ADMINISTRATION
● adults and children 12 years of age and older:
● apply at least a 1-inch strip of the product onto a soft bristle toothbrush.
● brush teeth thoroughly for at least 1 minute twice a day (morning and evening),
and not more than 3 times a day, or as recommended by a dentist or doctor.
Make sure to brush all sensitive areas of the teeth. Minimize swallowing. Spit out
after brushing.
● children under 12 years of age: Consult a dentist or doctor. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACNE TREATMENT CREAM
acne treatment cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84117-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 10 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) EDETATE DISODIUM (UNII: 7FLD91C86K) LAURETH-4 (UNII: 6HQ855798J) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYALURONIC ACID (UNII: S270N0TRQY) NIACINAMIDE (UNII: 25X51I8RD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84117-012-01 50 g in 1 CANISTER; Type 0: Not a Combination Product 07/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 07/03/2024 Labeler - Shenzhen Hengkaifeng Commerce and Trade Co., Ltd (444722774) Establishment Name Address ID/FEI Business Operations Shenzhen Hengkaifeng Commerce and Trade Co., Ltd 444722774 manufacture(84117-012) , label(84117-012)