Label: SODIUM SULFACETAMIDE AND SULFUR- sodium sulfacetamide 9.8% and sulfur 4.8% emulsion
- NDC Code(s): 44523-748-10
- Packager: Bio Comp Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
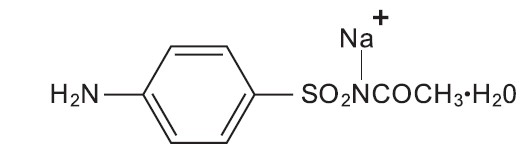
SPL UNCLASSIFIED SECTIONSODIUM SULFACETAMIDE 9.8% AND SULFUR 4.8% CLEANSER - (sodium sulfacetamide 9.8%, sulfur 4.8%) Rx Only - FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC - USE.
-
DESCRIPTION:
Each gram contains 98 mg of sodium sulfacetamide and 48 mg of colloidal sulfur in a vehicle consisting of: benzyl alcohol, cetyl alcohol, fragrance, glyceryl stearate (and) PEG-100 stearate ...
-
CLINICAL PHARMACOLOGY:
Sodium sulfacetamide exerts a bacteriostatic effect against sulfonamide sensitive Gram-positive and Gram-negative microorganisms commonly isolated from secondary cutaneous pyogenic infections. It ...
-
INDICATIONS:
This product is indicated for use in the topical control of acne vulgaris, acne rosacea and seborrheic dermatitis.
-
CONTRAINDICATIONS:
This product is contraindicated in persons with known or suspected hypersensitivity to any of the ingredients of the product. This product is not to be used by patients with kidney disease.
-
WARNINGS:
Sulfonamides are known to cause Stevens-Johnson syndrome in hypersensitive individuals. Stevens-Johnson syndrome also has - been reported following the use of sodium sulfacetamide topically ...
-
PRECAUTIONS:
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE. General: Nonsusceptible organisms, including fungi, may proliferate with the use of this preparation. Although rare, sensitivity to sodium ...
-
ADVERSE REACTIONS:
Reports of irritation and hypersensitivity to sodium sulfacetamide are uncommon. The following adverse reactions, reported - after administration of sterile ophthalmic sodium sulfacetamide, are ...
-
OVERDOSAGE:
The oral LD - 50 of sulfacetamide in mice is 16.5 g/kg. In the event of overdosage, emergency treatment should be started immediately. Manifestations: Overdosage may cause nausea and ...
-
DOSAGE AND ADMINISTRATION:
Shake well before using. Wash affected areas once or twice daily, or as directed by your physician. Wet skin and liberally apply - to areas to be cleansed. Massage gently into skin for 10 to 20 ...
-
STORAGE:
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). Brief exposure to - temperatures up to 40°C (104°F) may be tolerated provided the mean ...
-
NOTICE:
Protect from freezing and excessive heat. The product may tend to darken slightly on storage. Slight discoloration does not impair the efficacy or safety of the product. Keep bottle tightly ...
-
HOW SUPPLIED:
This product is supplied in the following size(s): 10 oz. (285 g) bottles, NDC 44523-748-10
-
SPL UNCLASSIFIED SECTIONTo report a serious adverse event or obtain product information, call 1-866-762-2365.
-
SPL UNCLASSIFIED SECTIONBCP_T13617R0424 - L74810R0224
-
PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information