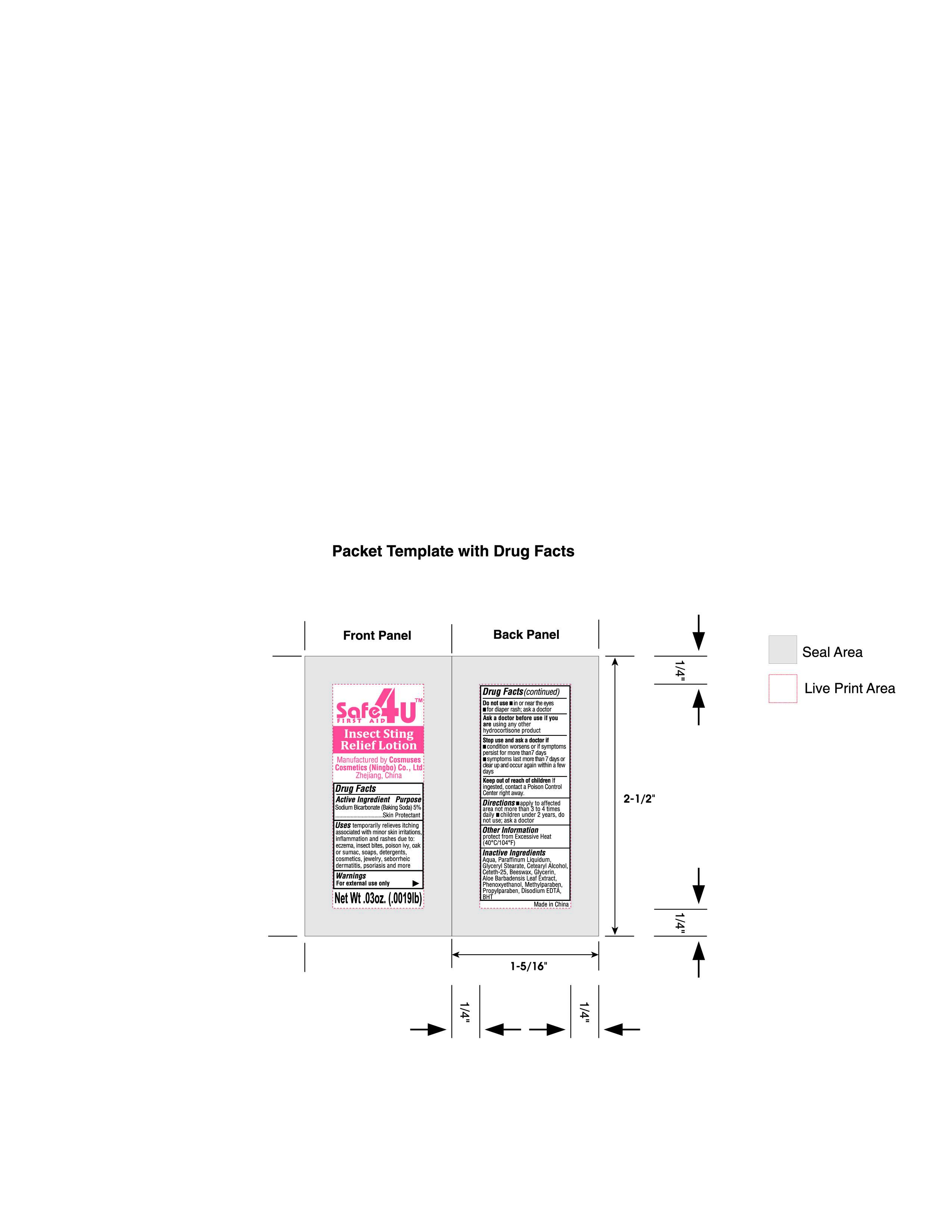
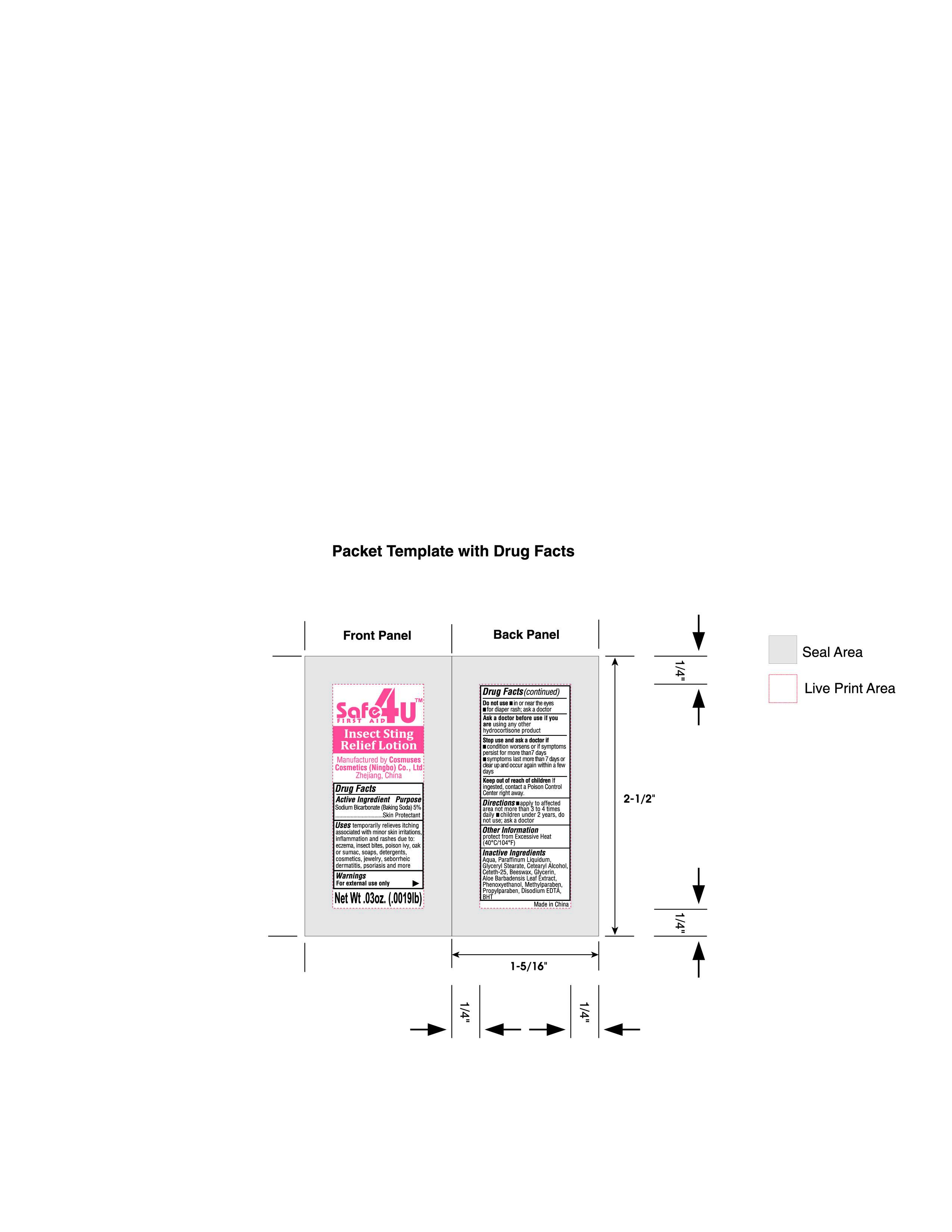
Label: INSECT STING RELIEF LATEX 01- sodium bicarbonate lotion
- NDC Code(s): 82953-020-01
- Packager: Cosmuses Cosmetics (Ningbo) Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- STORAGE AND HANDLING
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INSECT STING RELIEF LATEX 01
sodium bicarbonate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82953-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) (BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PROPYLPARABEN (UNII: Z8IX2SC1OH) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) CETETH-25 (UNII: 5KLY4IOG20) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82953-020-01 0.9 g in 1 POUCH; Type 1: Convenience Kit of Co-Package 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/14/2024 Labeler - Cosmuses Cosmetics (Ningbo) Co., Ltd. (725290934) Registrant - Cosmuses Cosmetics (Ningbo) Co., Ltd. (725290934) Establishment Name Address ID/FEI Business Operations Cosmuses Cosmetics (Ningbo) Co., Ltd. 725290934 manufacture(82953-020)