TERAZOSIN CAPSULES, USP
-
Rx only
-
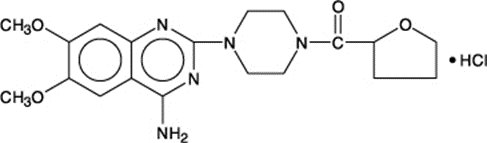
Generic Name: Terazosin (ter-A-so-sin)
When used to treat HYPERTENSION or BENIGN PROSTATIC HYPERPLASIA (BPH)
Please read this leaflet before you start ...
TERAZOSIN CAPSULES, USP
Rx only
Generic Name: Terazosin (ter-A-so-sin)
When used to treat HYPERTENSION or BENIGN PROSTATIC HYPERPLASIA (BPH)
Please read this leaflet before you start taking terazosin capsules. Also, read it each time you get a new prescription. This is a summary and should NOT take the place of a full discussion with your doctor who has additional information about terazosin capsules. You and your doctor should discuss terazosin capsules and your condition before you start taking it and at your regular checkups.
Terazosin capsules are used to treat high blood pressure (hypertension). Terazosin capsules are also used to treat benign prostatic hyperplasia (BPH) in men. This leaflet describes terazosin capsules as a treatment for hypertension or BPH.
What is hypertension (high blood pressure)?
Blood pressure is the tension of the blood within the blood vessels. If blood is pumped too forcefully, or if the blood vessels are too narrow, the pressure of the blood against the walls of the vessels rises.
If high blood pressure is not treated, over time, the increased pressure can damage blood vessels or it can cause the heart to work too hard and may decrease the flow of blood to the heart, brain, and kidneys. As a result, these organs may become damaged and not function correctly. If high blood pressure is controlled, this damage is less likely to happen.
Treatment options for hypertension
Non-drug treatments are sometimes effective in controlling mild hypertension. The most important lifestyle changes to lower blood pressure are to lose weight, reduce salt, fat, and alcohol in the diet, quit smoking, and exercise regularly. However, many hypertensive patients require one or more ongoing medications to control their blood pressure. There are different kinds of medications used to treat hypertension. Your doctor has prescribed terazosin capsules for you.
What terazosin capsules does to treat hypertension
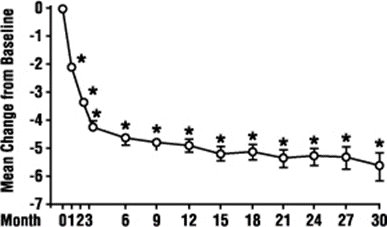
Terazosin capsules work by relaxing blood vessels so that blood passes through them more easily. This helps to lower blood pressure.
What is BPH?
The prostate is a gland located below the bladder of men. It surrounds the urethra (you-REETH-rah), which is a tube that drains urine from the bladder. BPH is an enlargement of the prostate gland. The symptoms of BPH, however, can be caused by an increase in the tightness of muscles in the prostate. If the muscles inside the prostate tighten, they can squeeze the urethra and slow the flow of urine. This can lead to symptoms such as:
- a weak or interrupted stream when urinating
- a feeling that you cannot empty your bladder completely
- a feeling of delay when you start to urinate
- a need to urinate often, especially at night, or
- a feeling that you must urinate right away.
Treatment options for BPH
There are three main treatment options for BPH:
- Program of monitoring or “Watchful Waiting”. Some men have an enlarged prostate gland, but no symptoms, or symptoms that are not bothersome. If this applies, you and your doctor may decide on a program of monitoring including regular checkups, instead of medication or surgery.
- Medication. There are different kinds of medication used to treat BPH. Your doctor has prescribed terazosin capsules for you. See “What terazosin capsules do to treat BPH” below.
- Surgery. Some patients may need surgery. Your doctor can describe several different surgical procedures to treat BPH. Which procedure is best depends on your symptoms and medical condition.
What terazosin capsules do to treat BPH
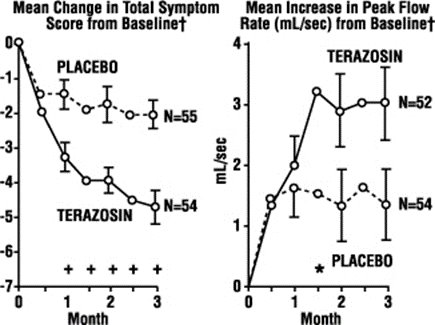
Terazosin capsules relax the tightness of a certain type of muscle in the prostate and at the opening of the bladder. This may increase the rate of urine flow and/or decrease the symptoms you are having.
- Terazosin capsules help relieve the symptoms of BPH. It does NOT change the size of the prostate, which may continue to grow. However, a larger prostate does not necessarily cause more or worse symptoms.
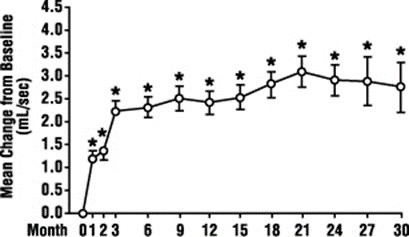
- If terazosin capsules are helping you, you should notice an effect on your particular symptoms in 2 to 4 weeks of starting to take the medication.
- Even though you take terazosin capsules and they may help you, terazosin capsules may not prevent the need for surgery in the future.
Other important facts about terazosin capsules for BPH
- You should see an effect on your symptoms in 2 to 4 weeks. So, you will need to continue seeing your doctor to check your progress regarding your BPH and to monitor your blood pressure in addition to your other regular checkups.
- Your doctor has prescribed terazosin capsules for your BPH and not for prostate cancer. However, a man can have BPH and prostate cancer at the same time. Doctors usually recommend that men be checked for prostate cancer once a year when they turn 50 (or 40 if a family member has had prostate cancer). These checks should continue even if you are taking terazosin capsules. Terazosin capsules are not a treatment for prostate cancer.
- About Prostate Specific Antigen (PSA). Your doctor may have done a blood test called PSA. Your doctor is aware that terazosin capsules do not affect PSA levels. You may want to ask your doctor more about this if you have had a PSA test done.
What you should know while taking terazosin capsules for hypertension or BPH
WARNINGS
Terazosin Capsules, USP Can Cause a Sudden Drop in Blood Pressure After the VERY FIRST DOSE.You may feel dizzy, faint, or “light-headed” particularly after you get up from bed or from a chair. This is more likely to occur after you’ve taken the first few doses, but can occur at any time while you are taking the drug. It can also occur if you stop taking the drug and then re-start treatment.
Because of this effect, your doctor may have told you to take terazosin capsules at bedtime. If you take terazosin capsules at bedtime but need to get up from bed to go to the bathroom, get up slowly and cautiously until you are sure how the medicine affects you. It is also important to get up slowly from a chair or bed at any time until you learn how you react to terazosin capsules. You should not drive or do any hazardous tasks until you are used to the effects of the medication. If you begin to feel dizzy, sit or lie down until you feel better.
- You will start with a 1 mg dose of terazosin capsules. Then the dose will be increased as your body gets used to the effect of the medication.
- Other side effects you could have while taking terazosin capsules include drowsiness, blurred or hazy vision, nausea, or “puffiness” of the feet or hands. Discuss any unexpected effects you notice with your doctor.
Extremely rarely, terazosin capsules and similar medications have caused painful erection of the penis, sustained for hours and unrelieved by sexual intercourse or masturbation. This condition is serious, and if untreated it can be followed by permanent inability to have an erection. If you have a prolonged abnormal erection, call your doctor or go to an emergency room as soon as possible.
How to take terazosin capsules
Follow your doctor’s instructions about how to take terazosin capsules. You must take it every day at the dose prescribed. Talk with your doctor if you don’t take it for a few days, you may have to restart it at a 1 mg dose and be cautious about possible dizziness. Do not share terazosin capsules with anyone else; it was prescribed only for you.
Keep terazosin capsules and all medicines out of the reach of children.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Protect from light and moisture.
FOR MORE INFORMATION ABOUT TERAZOSIN CAPSULES AND HYPERTENSION OR BPH, TALK WITH YOUR DOCTOR, NURSE, PHARMACIST OR OTHER HEALTH CARE PROVIDER.
To report SUSPECTED ADVERSE REACTIONS, contact Apnar Pharma LP. at 1-855-642-2594 or email safety.apnarpharma@lambda-cro.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manufactured for:
Apnar Pharma LP
Chino, CA, USA 91710
Rev. 12/2022
Marketed by:
GSMS, Inc.
Camarillo, CA 93012 USA
Close