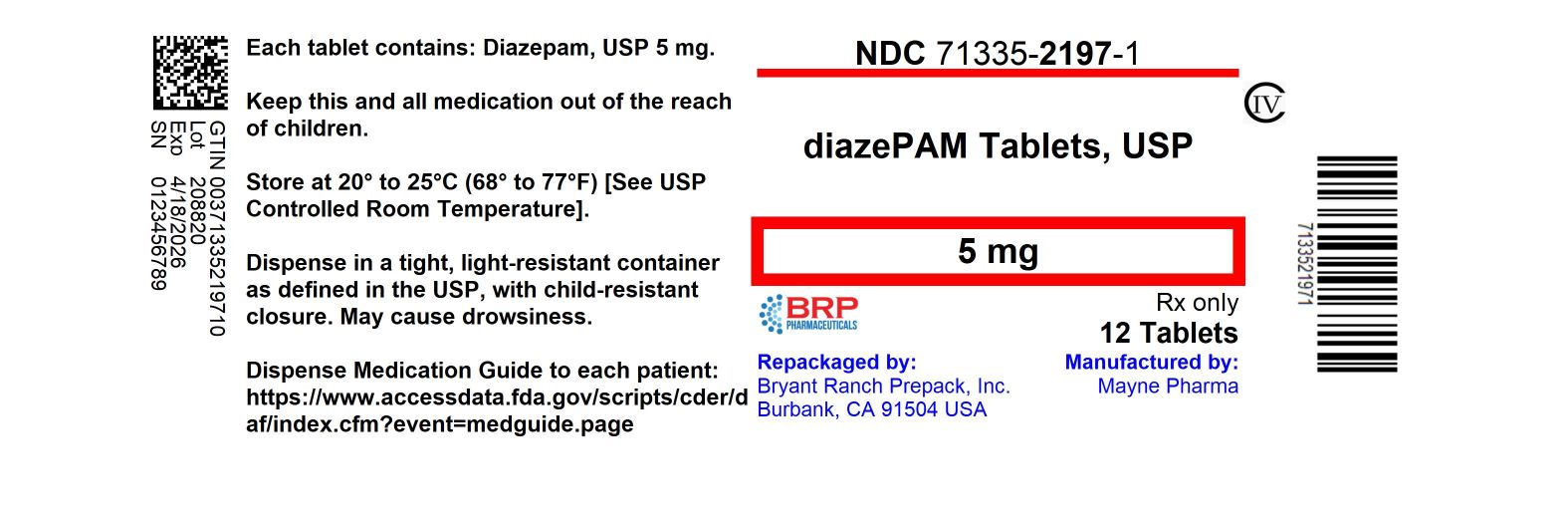
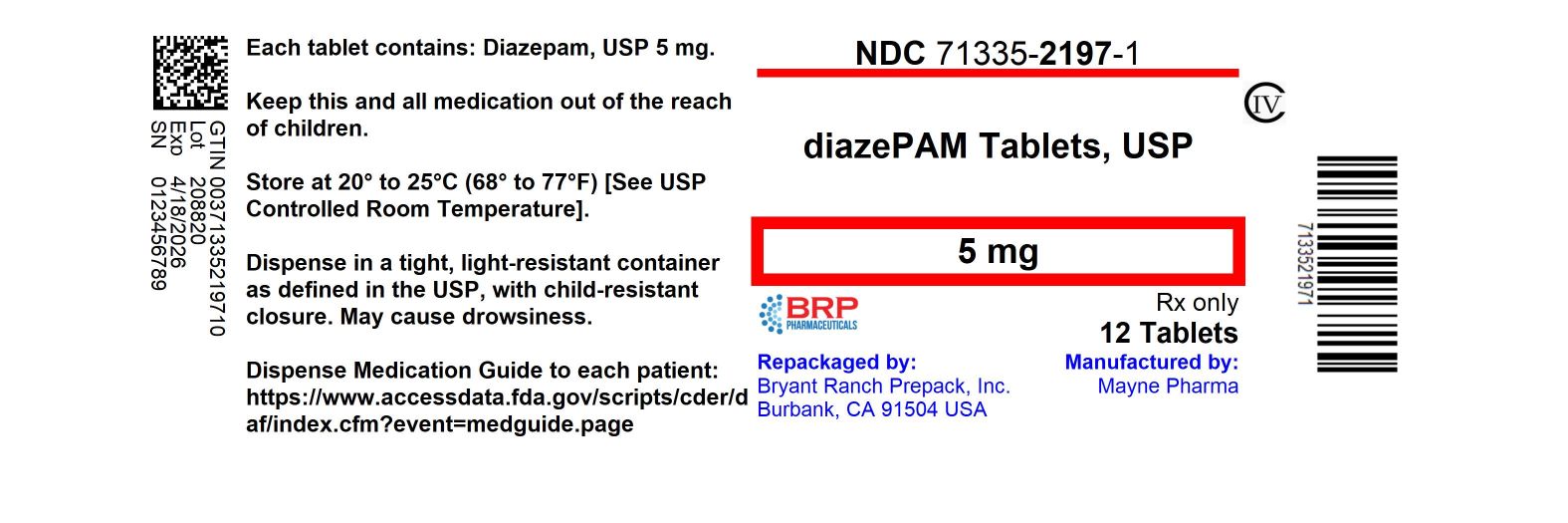
Label: DIAZEPAM tablet
- NDC Code(s): 71335-2197-0, 71335-2197-1, 71335-2197-2, 71335-2197-3, view more
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 51862-942
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
BOXED WARNING
(What is this?)
WARNINING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation (see WARNINGSand PRECAUTIONS).
- The use of benzodiazepines, including Diazepam, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing Diazepam and throughout treatment, assess each patient's risk for abuse, misuse, and addiction (see WARNINGS) .
- The continued use of benzodiazepines, including Diazepam, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of Diazepam after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Diazepam or reduce the dosage (see DOSAGE AND ADMINISTRATIONand WARNINGS)
-
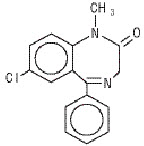
DESCRIPTIONDiazepam is a benzodiazepine derivative. The chemical name of diazepam is 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one. It is a colorless to light yellow crystalline compound ...
-
CLINICAL PHARMACOLOGYDiazepam is a benzodiazepine that exerts anxiolytic, sedative, muscle-relaxant, anticonvulsant and amnestic effects. Most of these effects are thought to result from a facilitation of the action ...
-
INDICATIONSDiazepam Tablets USP are indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life ...
-
CONTRAINDICATIONSDiazepam Tablets USP are contraindicated in patients with a known hypersensitivity to this drug and, because of lack of sufficient clinical experience, in pediatric patients under 6 months of age ...
-
WARNINGSRisks from Concomitant Use with Opioids - Concomitant use of benzodiazepines, including diazepam tablets, and opioids may result in profound sedation, respiratory depression, coma, and death ...
-
PRECAUTIONSGeneral - If diazepam is to be combined with other psychotropic agents or anticonvulsant drugs, careful consideration should be given to the pharmacology of the agents to be employed ...
-
ADVERSE REACTIONSSide effects most commonly reported were drowsiness, fatigue, muscle weakness, and ataxia. The following have also been reported: Central Nervous System:confusion, depression, dysarthria ...
-
DRUG ABUSE AND DEPENDENCEControlled Substance - Diazepam is a Schedule IV controlled substance. Abuse - Diazepam is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the ...
-
OVERDOSAGEOverdose of benzodiazepines is usually manifested by central nervous system depression ranging from drowsiness to coma. In mild cases, symptoms include drowsiness, confusion, and lethargy. In more ...
-
DOSAGE AND ADMINISTRATIONDosage should be individualized for maximum beneficial effect. While the usual daily dosages given below will meet the needs of most patients, there will be some who may require higher doses. In ...
-
HOW SUPPLIEDDiazepam Tablets USP 5 mg are yellow, flat-faced, bevel edge, bisected tablets imprinted MYX/942 on one side and 5 imprinted on the other side. NDC: 71335-2197-1: 12 Tablets in a BOTTLE - NDC ...
-
SPL UNCLASSIFIED SECTIONMedication Guides available at products.maynepharma.com or call 1-844-825-8500. Manufactured by: Mayne Pharma - Greenville, NC 27834 - Revised: 03/2021 - 61771
-
MEDICATION GUIDEMEDICATION GUIDE - DIAZEPAM (dye az' e pam) TABLETS, C-IV - What is the most important information I should know about Diazepam Tablets? Diazepam tablets are a ...
-
PRINCIPAL DISPLAY PANELDiazepam 5mg (CIV) Tablet
-
INGREDIENTS AND APPEARANCEProduct Information