Label: DERMAZINC- pyrithione zinc soap
- NDC Code(s): 35324-004-04
- Packager: WynnPharm Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
-
WARNINGS
Warnings
■ Ask a doctor before use if you have a condition that covers a large area of the body.When using this product avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with
water.
Stop use and ask a doctor if condition worsens or does not improve after regular use of this
product as directed.If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.■ For external use only.
- INACTIVE INGREDIENT
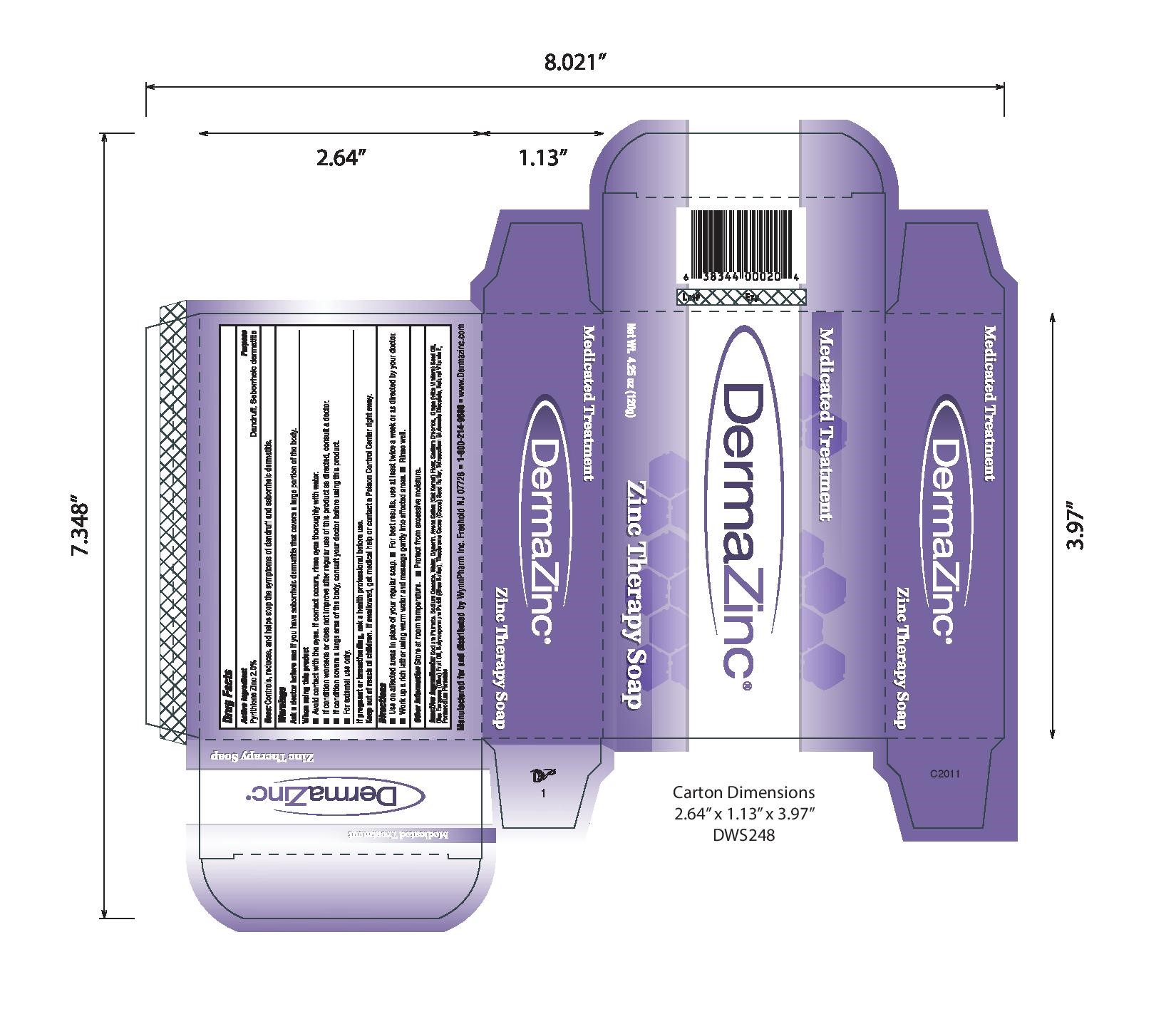
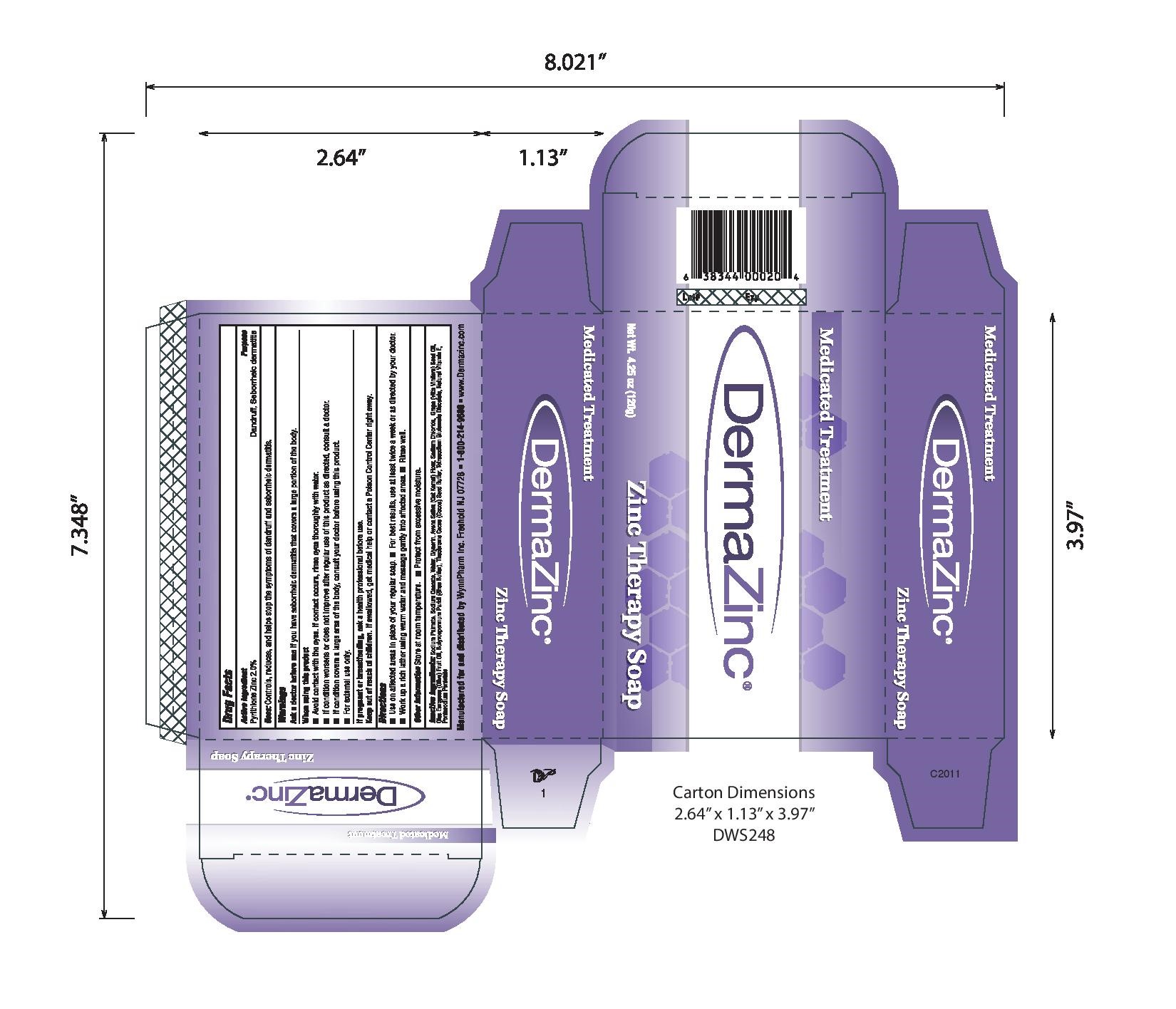
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DERMAZINC
pyrithione zinc soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:35324-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 2 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) AVENA SATIVA WHOLE (UNII: 5P8D0Z74RG) SODIUM CHLORIDE (UNII: 451W47IQ8X) PENTASODIUM PENTETATE (UNII: 961TOZ5L7T) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) SHEA BUTTER (UNII: K49155WL9Y) VITAMIN E POLYETHYLENE GLYCOL SUCCINATE (UNII: O03S90U1F2) OLIVE OIL (UNII: 6UYK2W1W1E) GRAPE SEED OIL (UNII: 930MLC8XGG) SODIUM PALMATE (UNII: S0A6004K3Z) SODIUM PALM KERNELATE (UNII: 6H91L1NXTW) WATER (UNII: 059QF0KO0R) COCOA BUTTER (UNII: 512OYT1CRR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35324-004-04 120 g in 1 BOX; Type 0: Not a Combination Product 03/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 03/01/2024 Labeler - WynnPharm Inc (620885173) Registrant - WynnPharm Inc (620885173)