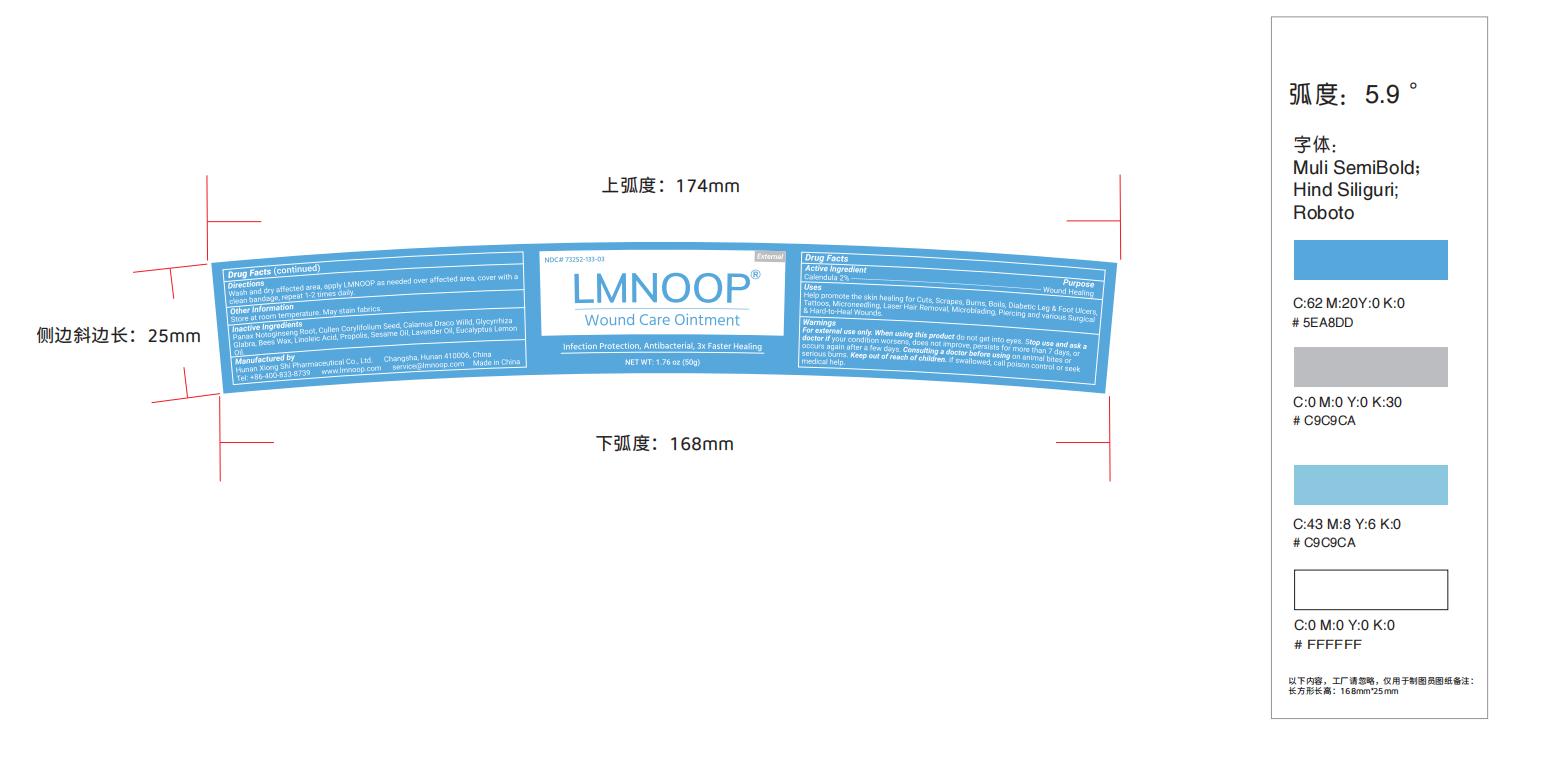
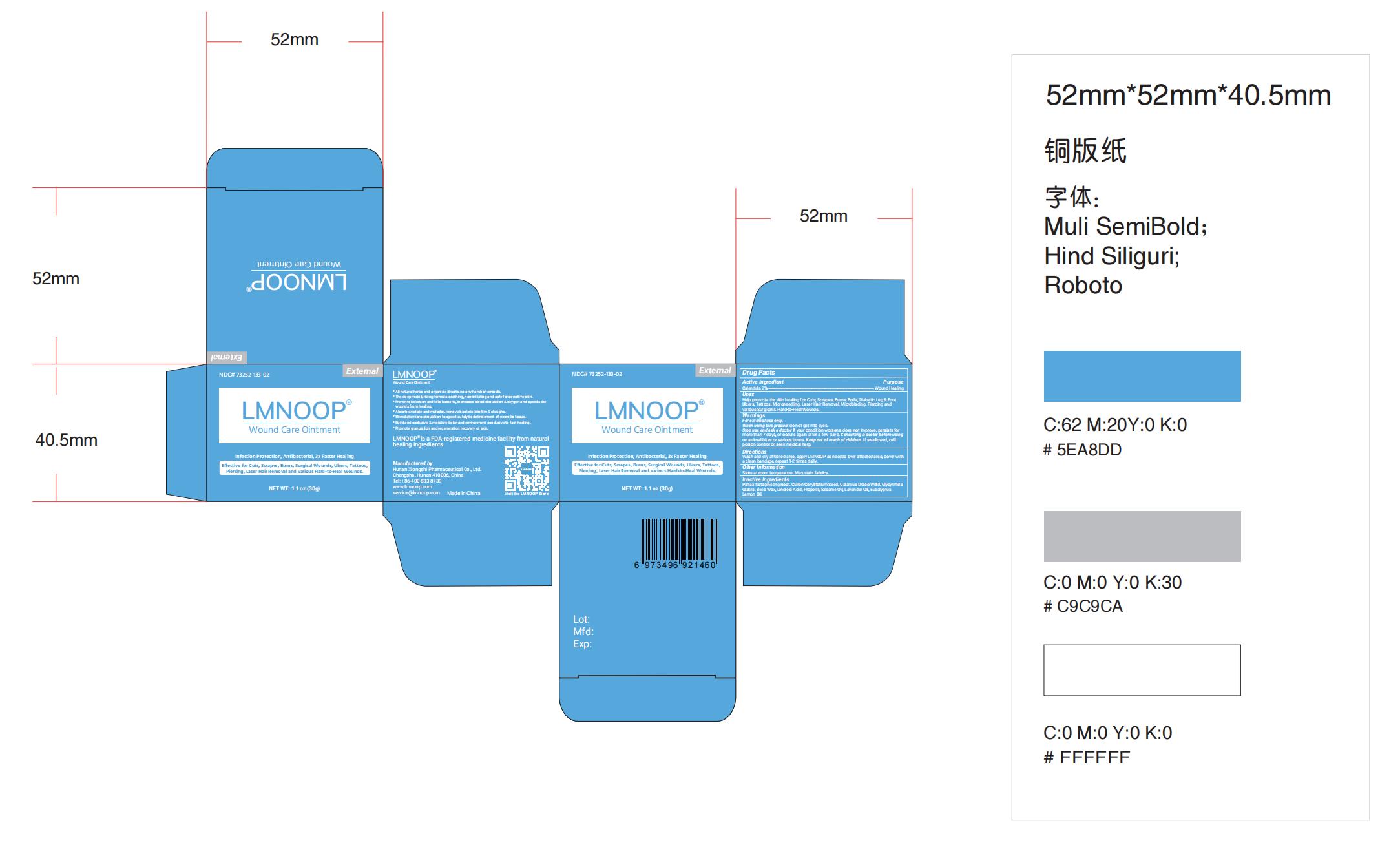
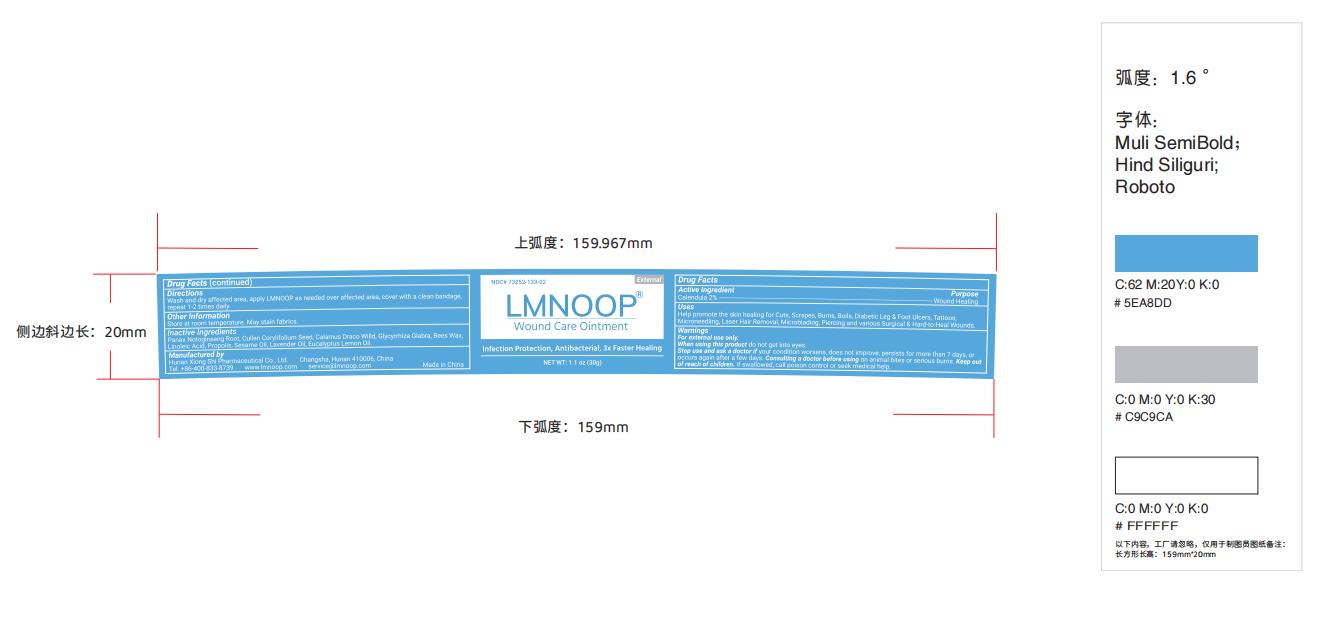
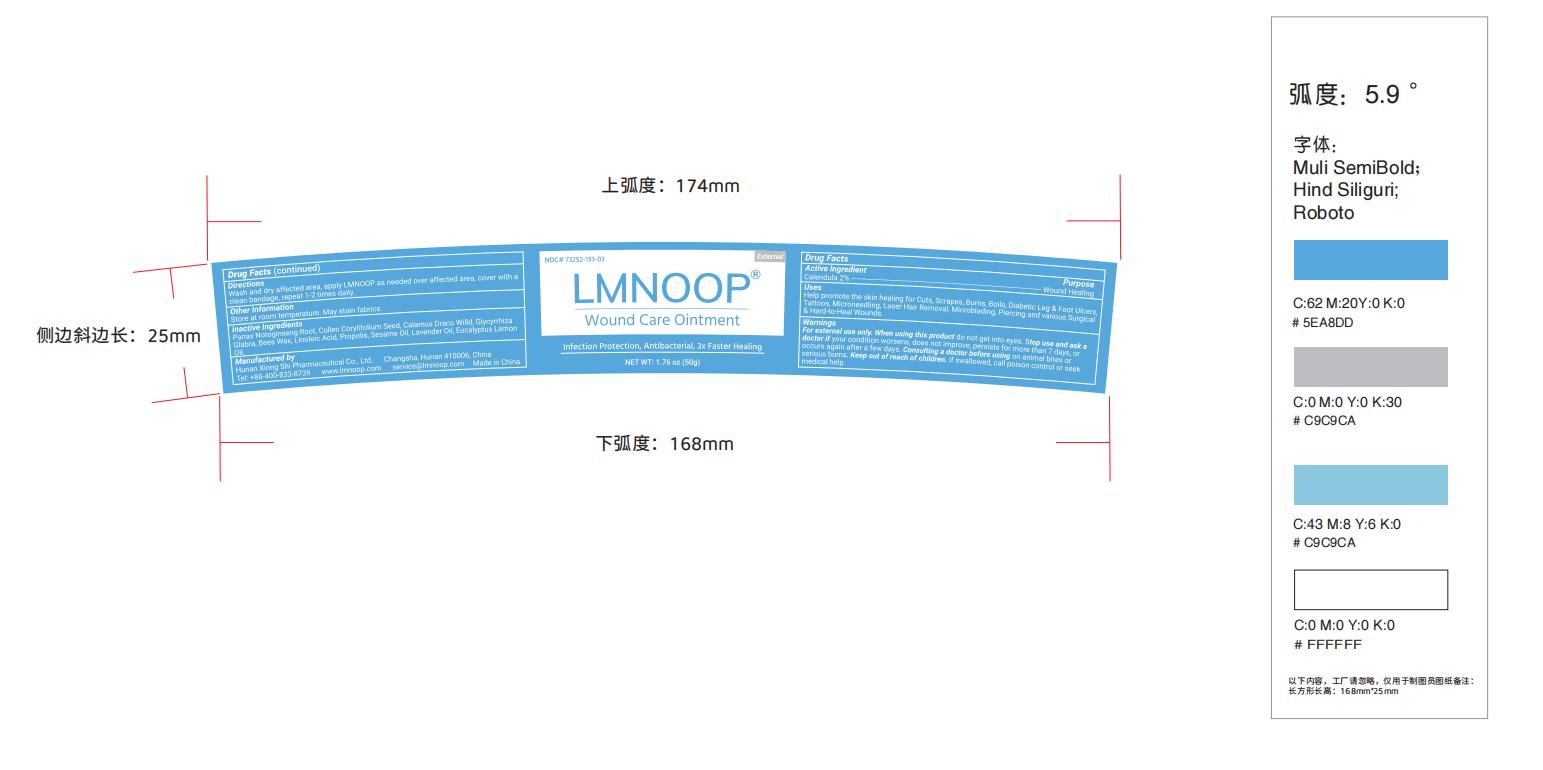
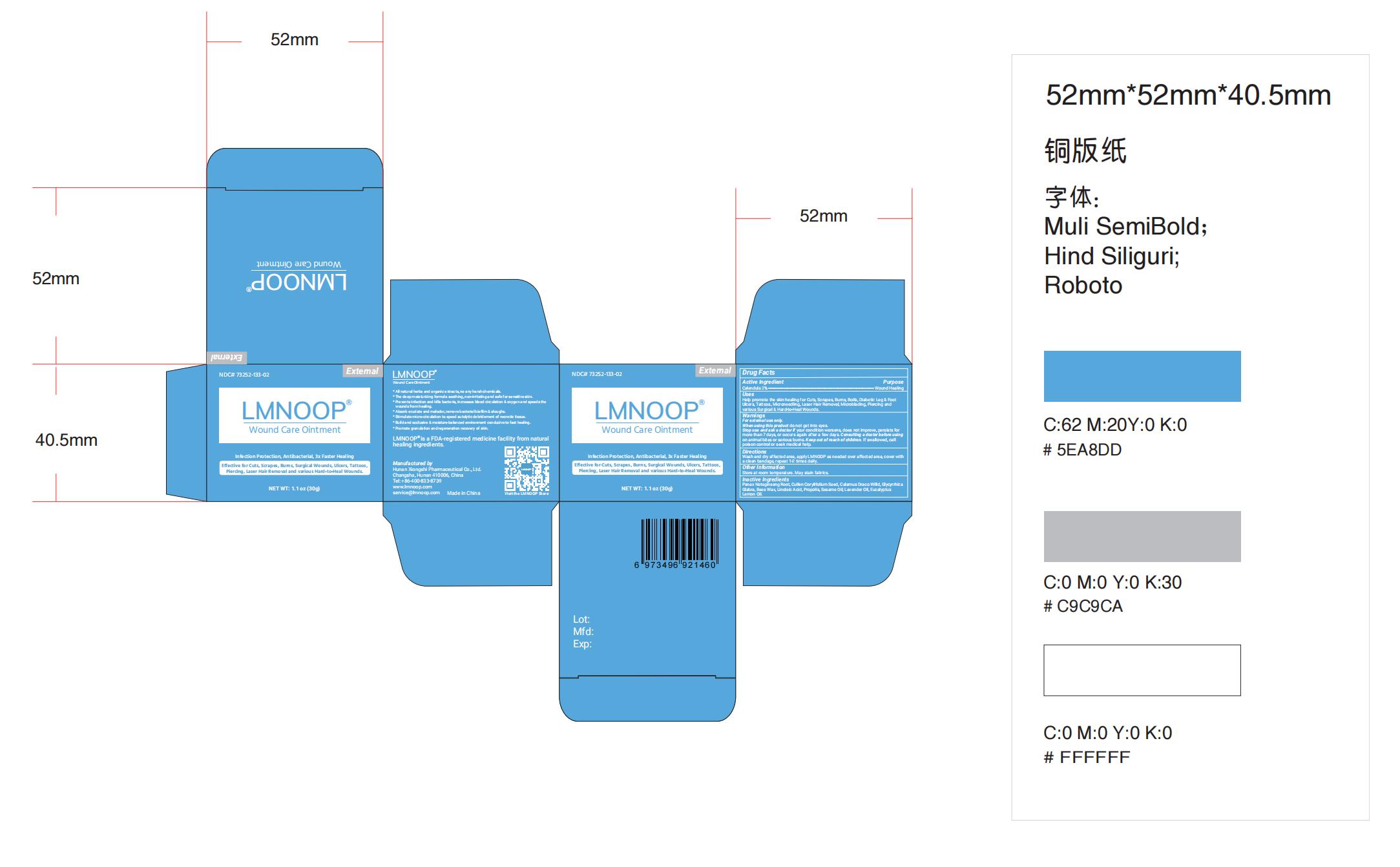
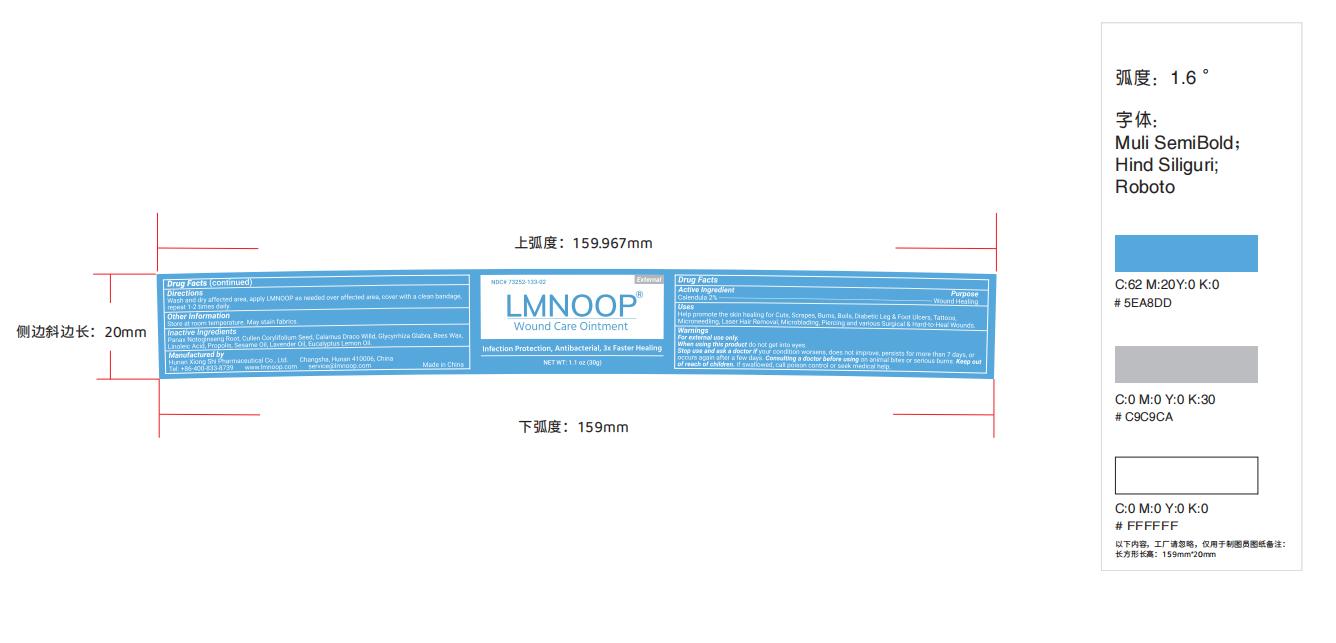
Label: LMNOOP WOUND CARE- wound care ointment ointment
- NDC Code(s): 73252-160-01, 73252-160-02, 73252-160-03
- Packager: Hunan Xiong Shi Pharmaceutical Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Inactive Ingredients
- Keep out of reach of children.
- Consulting a doctor before using
- Stop use and ask a doctor
- Purpose
- Other information
- Directions
- Dosage & administration
- When using this product
- Warnings
- Label
-
INGREDIENTS AND APPEARANCE
LMNOOP WOUND CARE
wound care ointment ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73252-160 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) (CALENDULA OFFICINALIS FLOWER - UNII:P0M7O4Y7YD) CALENDULA OFFICINALIS FLOWER 2 g in 100 g Inactive Ingredients Ingredient Name Strength GANODERMA LUCIDUM WHOLE (UNII: J5P04QW0CF) LINOLEIC ACID (UNII: 9KJL21T0QJ) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) ANGELICA SINENSIS ROOT (UNII: B66F4574UG) GLYCYRRHIZA URALENSIS (UNII: 42B5YD8F0K) CORYMBIA CITRIODORA LEAF OIL (UNII: M63U6N96EB) PROPOLIS WAX (UNII: 6Y8XYV2NOF) SESAME OIL (UNII: QX10HYY4QV) YELLOW WAX (UNII: 2ZA36H0S2V) LAVENDER OIL (UNII: ZBP1YXW0H8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73252-160-02 50 g in 1 BOTTLE; Type 0: Not a Combination Product 05/07/2019 2 NDC:73252-160-01 30 g in 1 BOTTLE; Type 0: Not a Combination Product 05/07/2019 3 NDC:73252-160-03 15 g in 1 TUBE; Type 0: Not a Combination Product 05/07/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 05/07/2019 Labeler - Hunan Xiong Shi Pharmaceutical Co., Ltd (554497403) Registrant - Hunan Xiong Shi Pharmaceutical Co., Ltd (554497403) Establishment Name Address ID/FEI Business Operations Hunan Xiong Shi Pharmaceutical Co., Ltd. 554497403 manufacture(73252-160)