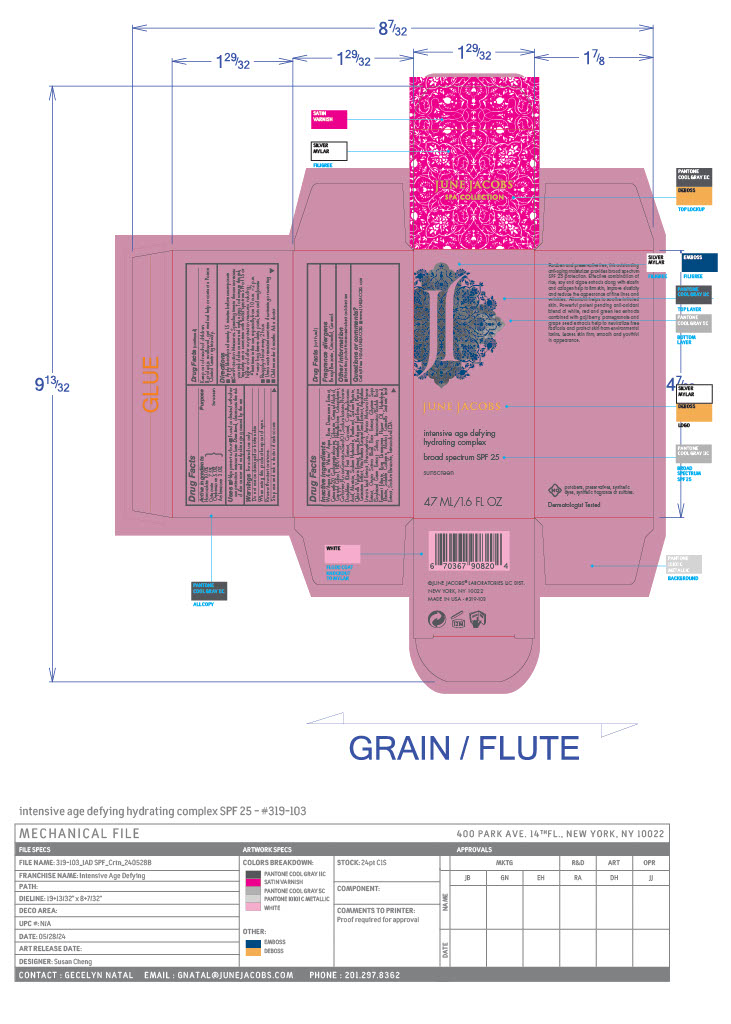
Label: INTENSIVE AGE DEFYING HYDRATING COMPLEX SPF 25- homosalate, octinoxate, oxybenzone, avobenzone cream
- NDC Code(s): 65278-103-01, 65278-103-02
- Packager: June Jacobs Labs, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions ■Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early aging. To decrease this risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m. – 2 p.m. • wear long sleeve shirts, pants, hats and sunglasses
■ Reapply at least every 2 hours ■ Use a water resistant sunscreen if swimming or sweating ■ Children under 6 months: Ask a doctor
-
INACTIVE INGREDIENT
Inactive ingredients
Water/Aqua/Eau, Water/ Aqua, Rosa Damascena Extract, Ceteareth-20, Cyclopentasiloxane, Glycerin, Cetearyl Alcohol, Caprylyl Glycol, HDI/Trimethylol Hexyllactone Crosspolymer, Carbomer, Punica Granatum Seed Oil, Sodium Lactate, Phoenix Dactylifera (Date) Fruit Extract, Caramel, Caprylhydroxamic Acid, Allantoin, Sodium Hydroxide, Panthenol, Sodium Phytate, Chlorella Vulgaris Extract, Lycium Barbarum Fruit Extract, Punica Granatum Extract, Vitis Vinifera (Grape) Seed Extract, Aspalathus Linearis Leaf Extract, Phenoxyethano, Arnica Montana Flower Extract, Oryza Sativa (Rice) Bran Extract, Glycine Soja (Soybean) Germ Extract, Silica, Leuconostoc/Radish Root Ferment Filtrate, Rosa Damascena Flower Oil, Hydrolyzed Elastin, Soluble Collagen, Alcohol, Camellia Sinensis Leaf Extract, Sodium Benzoate, Tetrasodium EDTA, Benzyl Benzoate, Citronellol, Geraniol.
- OTHER SAFETY INFORMATION
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INTENSIVE AGE DEFYING HYDRATING COMPLEX SPF 25
homosalate, octinoxate, oxybenzone, avobenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65278-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.549 g in 47.32 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1.4196 g in 47.32 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 4.732 g in 47.32 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 2.366 g in 47.32 g Inactive Ingredients Ingredient Name Strength PANTHENOL (UNII: WV9CM0O67Z) ASPALATHUS LINEARIS LEAF (UNII: H7UGK1GJCU) PHENOXYETHANOL (UNII: HIE492ZZ3T) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) RICE BRAN (UNII: R60QEP13IC) HYDROLYZED BOVINE ELASTIN (BASE; 1000 MW) (UNII: ZR28QKN0WT) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PHYTATE SODIUM (UNII: 88496G1ERL) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) LYCIUM BARBARUM FRUIT (UNII: 930626MWDL) ROSA DAMASCENA FLOWER OIL (UNII: 18920M3T13) ALCOHOL (UNII: 3K9958V90M) ROSA DAMASCENA FLOWERING TOP (UNII: 21W82Q764G) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PUNICA GRANATUM SEED OIL (UNII: 0UI45XV0T6) ALLANTOIN (UNII: 344S277G0Z) CARAMEL (UNII: T9D99G2B1R) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) EDETATE SODIUM (UNII: MP1J8420LU) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) GERANIOL (UNII: L837108USY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CHLORELLA VULGARIS (UNII: RYQ4R60M02) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) BENZYL BENZOATE (UNII: N863NB338G) SODIUM HYDROXIDE (UNII: 55X04QC32I) PUNICA GRANATUM ROOT BARK (UNII: CLV24I3T1D) VITIS VINIFERA SEED (UNII: C34U15ICXA) SOYBEAN GERM (UNII: JBW2VHD14M) COLLAGEN, SOLUBLE, FISH SKIN (UNII: 8JC99XGU4W) WATER (UNII: 059QF0KO0R) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM LACTATE, L- (UNII: P2Y1C6M9PS) DATE (UNII: H3O7QI5HY7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65278-103-01 47.32 g in 1 TUBE; Type 0: Not a Combination Product 10/23/2014 2 NDC:65278-103-02 47.32 g in 1 CARTON; Type 0: Not a Combination Product 10/23/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/23/2014 Labeler - June Jacobs Labs, LLC (082439410) Establishment Name Address ID/FEI Business Operations June Jacobs Labs, LLC 122610681 manufacture(65278-103)