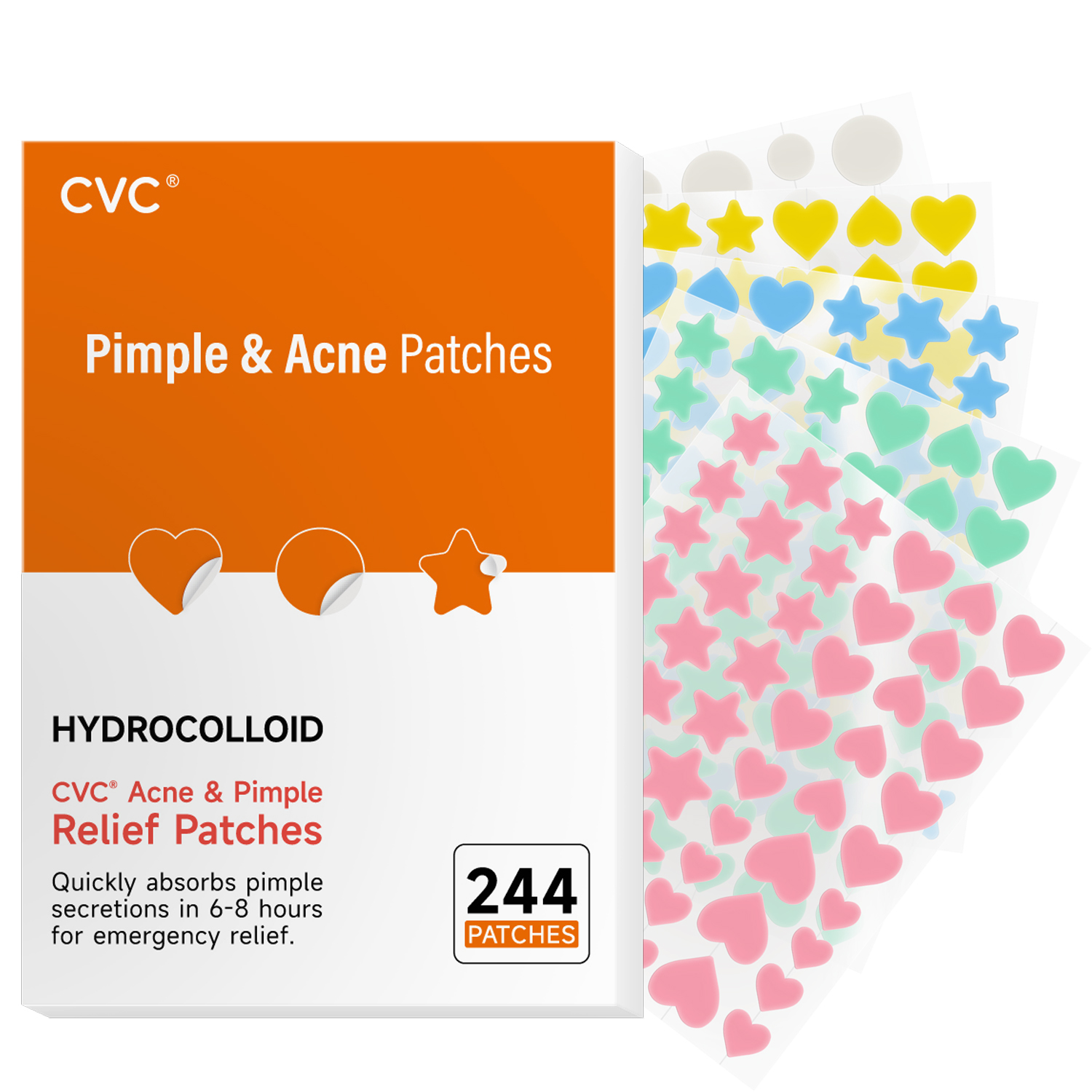
Label: CVC PIMPLE ACNE 244PATCHES- pimple acne patches patch
- NDC Code(s): 83872-251-01
- Packager: Shenzhen Xiaomai Manufacturing Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When using this product
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVC PIMPLE ACNE 244PATCHES
pimple acne patches patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83872-251 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength DIOCTYLDODECYL ADIPATE (UNII: 9MV77C2BPI) CENTELLA ASIATICA (UNII: 7M867G6T1U) TEA TREE OIL (UNII: VIF565UC2G) POLYURETHANE-34 (40 MPA, TENSILE STRENGTH OF FILM AT BREAK) (UNII: 77KA3O6NNF) DISODIUM CARBOXYMETHYLARSONATE (UNII: T9HTL93NB7) POLYETHYLENE TEREPHTHALATE (INTRINSIC VISCOSITY 0.60-0.70) (UNII: 5YSH70HE6K) HYDROGENATED POLYISOBUTENE 8 (UNII: 7YR4ZFS62E) GELATIN (UNII: 2G86QN327L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83872-251-01 20 g in 1 CARTON; Type 0: Not a Combination Product 05/18/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/18/2024 Labeler - Shenzhen Xiaomai Manufacturing Co., Ltd. (712999147) Establishment Name Address ID/FEI Business Operations Shenzhen Xiaomai Manufacturing Co., Ltd. 712999147 manufacture(83872-251)