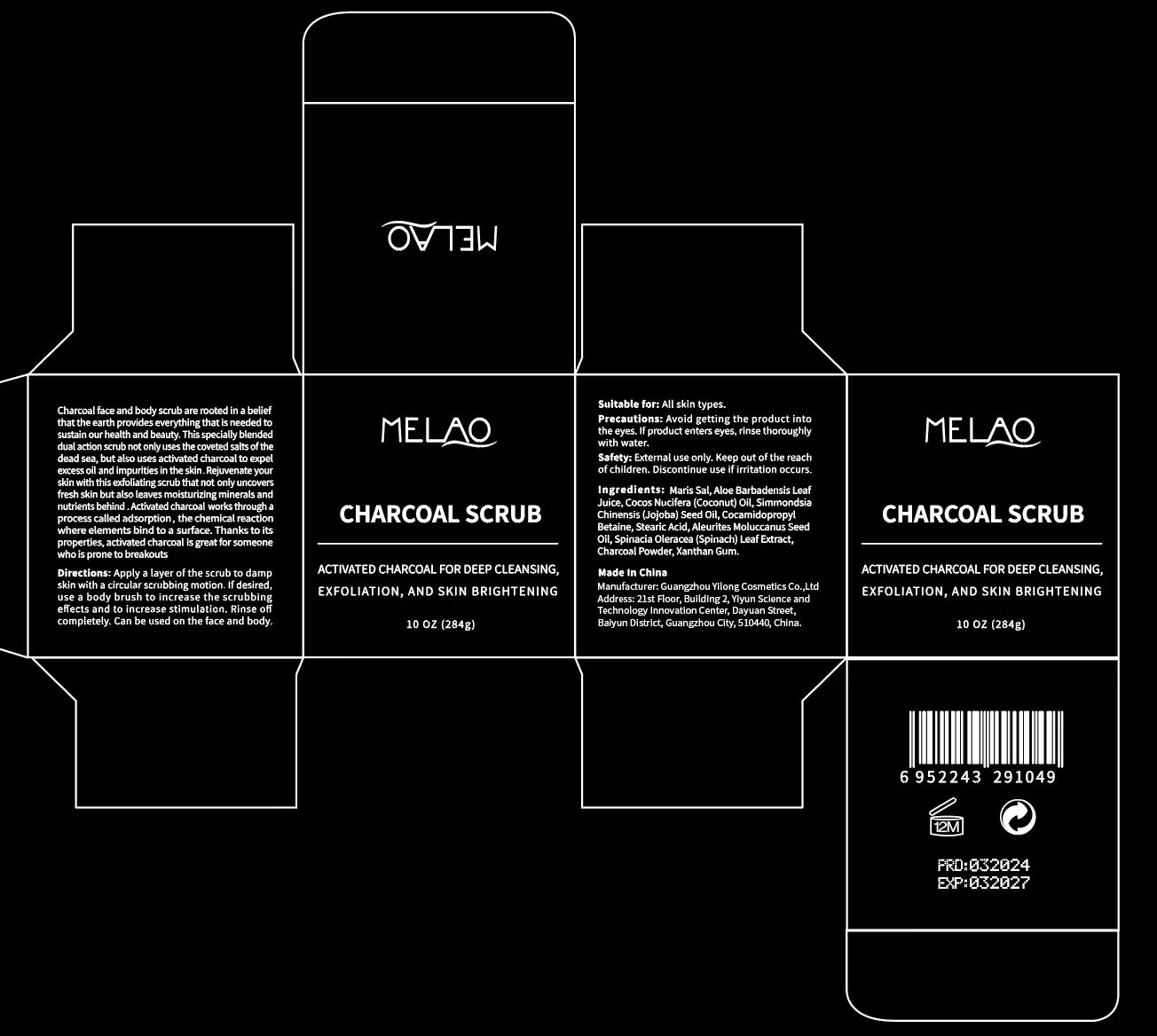
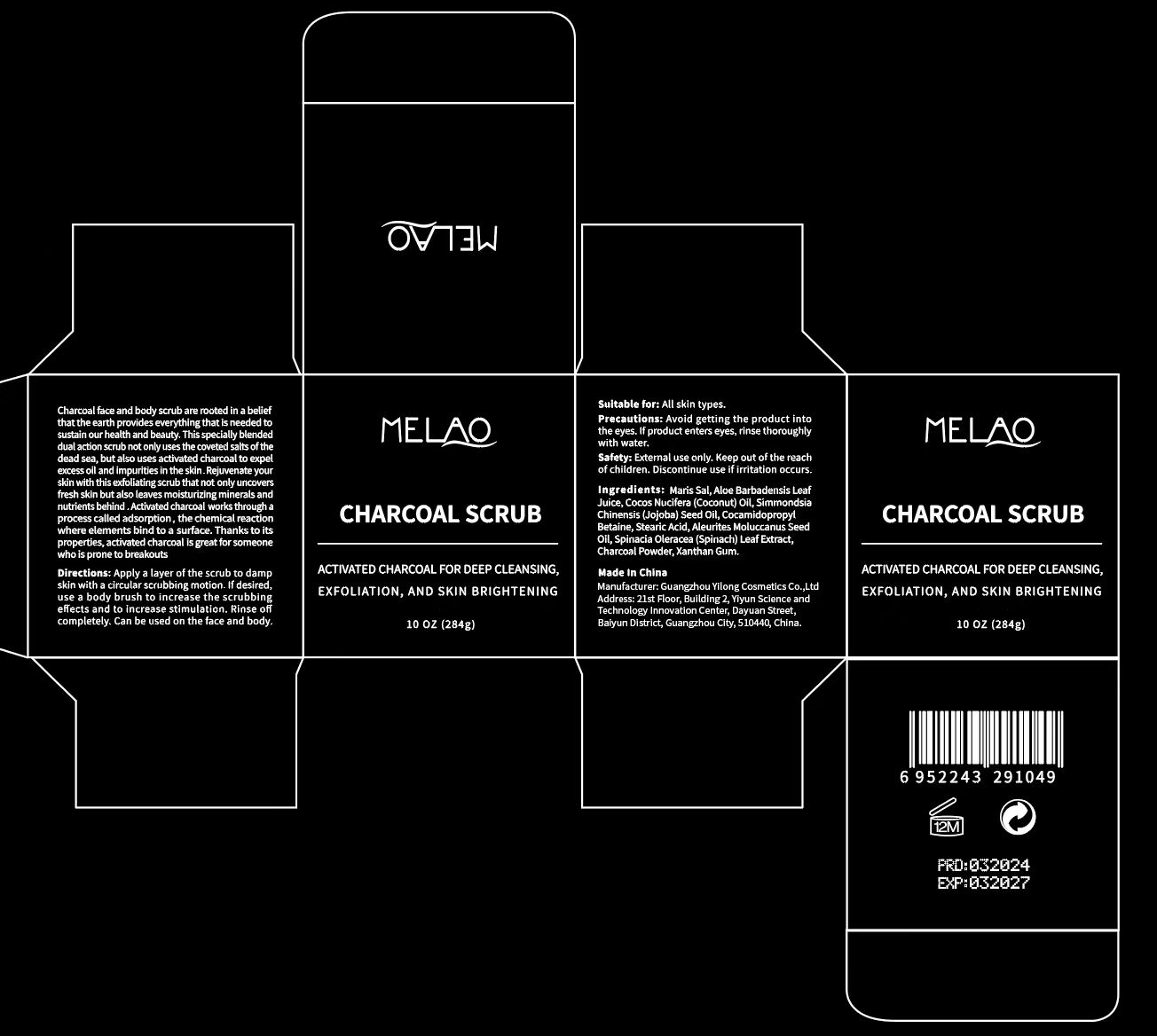
Label: MELAO CHARCOAL SCRUB- charcoal granule
- NDC Code(s): 83566-069-01
- Packager: Guangzhou Yilong Cosmetics Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- WARNINGS
- Stop use and ask a doctor if
- Do not use
- Do not use
- When using this product
- Keep out of reach of children.
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MELAO CHARCOAL SCRUB
charcoal granuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83566-069 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 4 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE ANDONGENSIS LEAF (UNII: N1P4NU25EJ) STEARIC ACID (UNII: 4ELV7Z65AP) SPINACIA OLERACEA WHOLE (UNII: 7UT47MP92R) SIMMONDSIA CHINENSIS SEED (UNII: D24K2Q1F6H) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) XANTHAN GUM (UNII: TTV12P4NEE) SEA SALT (UNII: 87GE52P74G) ALEURITES MOLUCCANA SEED (UNII: J87WJ3E7VW) COCOS NUCIFERA WHOLE (UNII: 245J88W96L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83566-069-01 284 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/20/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/20/2023 Labeler - Guangzhou Yilong Cosmetics Co.,Ltd. (712647107) Establishment Name Address ID/FEI Business Operations Guangzhou Yilong Cosmetics Co.,Ltd. 712647107 manufacture(83566-069)