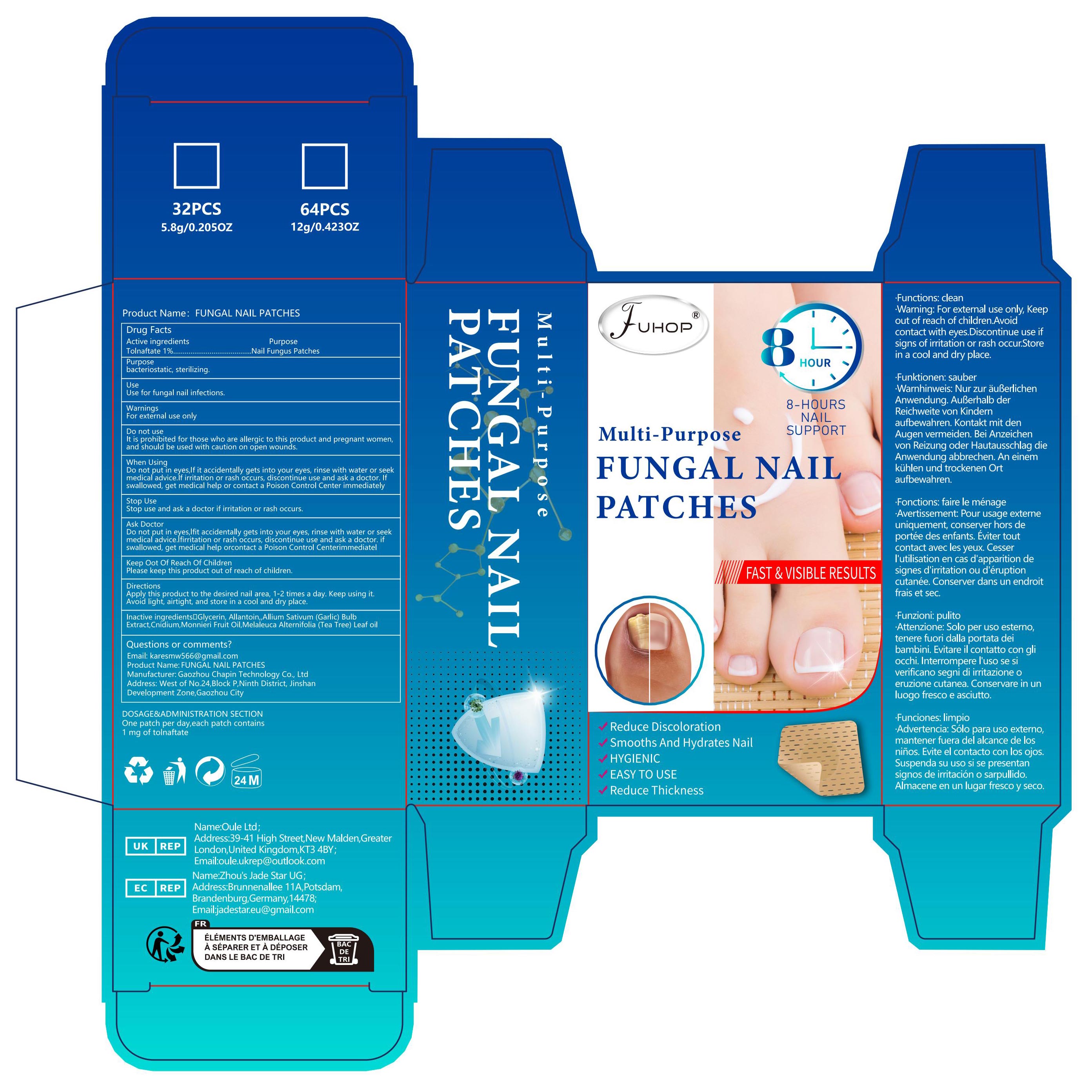
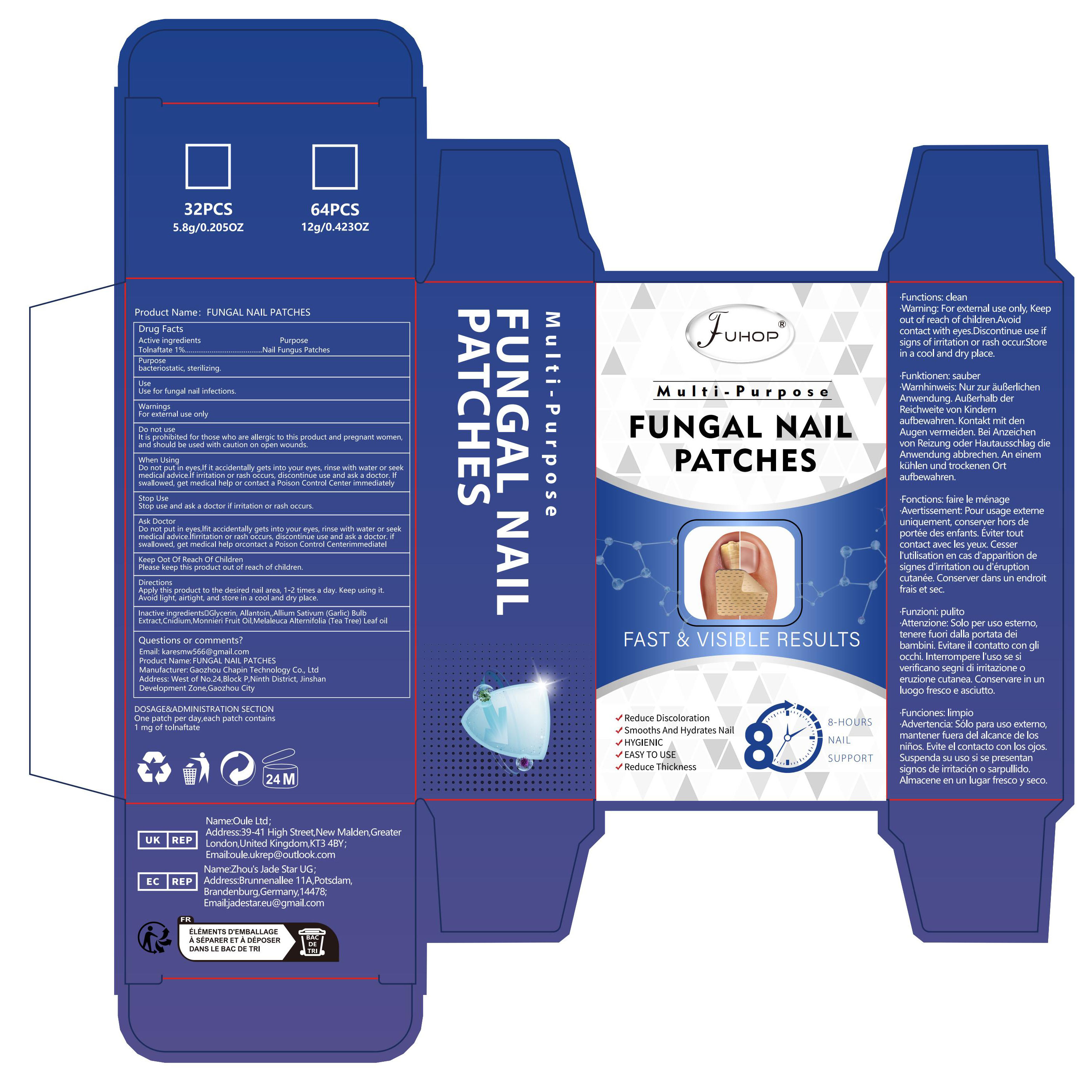
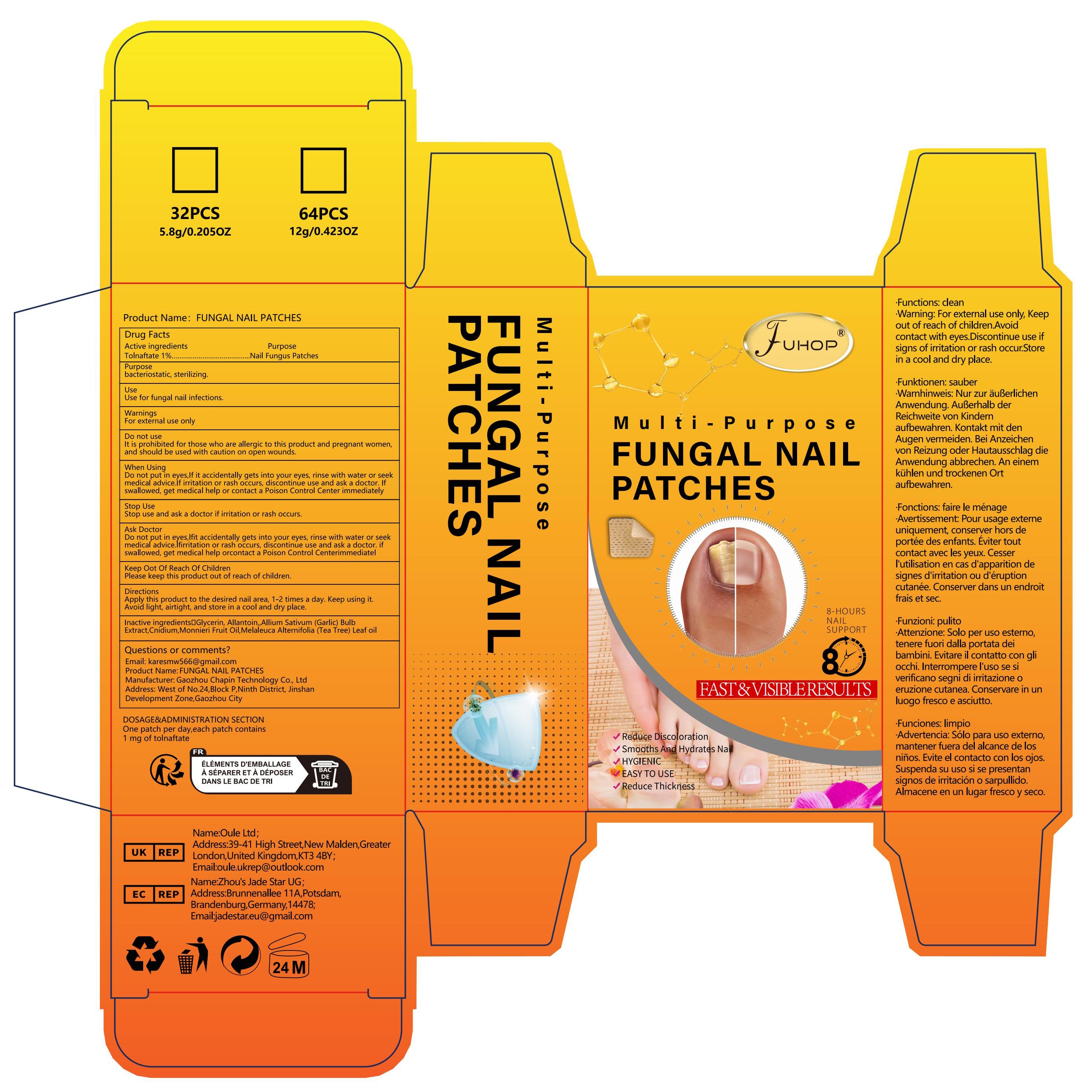
Label: FUNGAL NAIL PATCHES- fungal nail renew patch
- NDC Code(s): 84168-010-01
- Packager: Gaozhou Chapin Technology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- DOSAGE & ADMINISTRATION SECTION
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FUNGAL NAIL PATCHES
fungal nail renew patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84168-010 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength CNIDIUM MONNIERI FRUIT OIL (UNII: JK0MS9P8YL) 240 mg in 1 g GLYCERIN (UNII: PDC6A3C0OX) 160 mg in 1 g MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL (UNII: VIF565UC2G) 180 mg in 1 g GARLIC (UNII: V1V998DC17) 230 mg in 1 g ALLANTOIN (UNII: 344S277G0Z) 180 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84168-010-01 1 g in 1 BOX; Type 0: Not a Combination Product 04/06/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 04/06/2024 Labeler - Gaozhou Chapin Technology Co., Ltd (715573719) Establishment Name Address ID/FEI Business Operations Gaozhou Chapin Technology Co., Ltd 715573719 manufacture(84168-010)