Label: MUCINEX CHILDRENS MIGHTY CHEWS COUGH- dextromethorphan hydrobromide tablet, chewable
- NDC Code(s): 72854-081-16
- Packager: RB Health (US) LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- Uses
-
WARNINGS
Warnings
Do not use
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI)
(certain drugs for depression, psychiatric, or emotional conditions, or
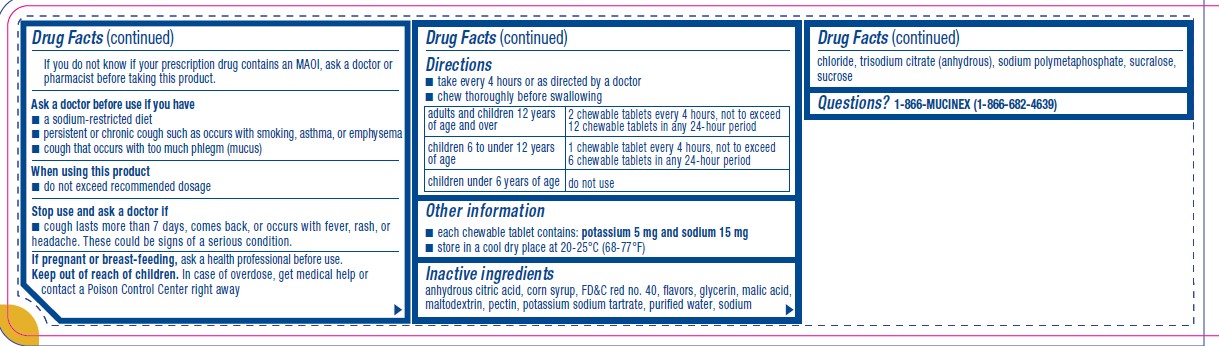
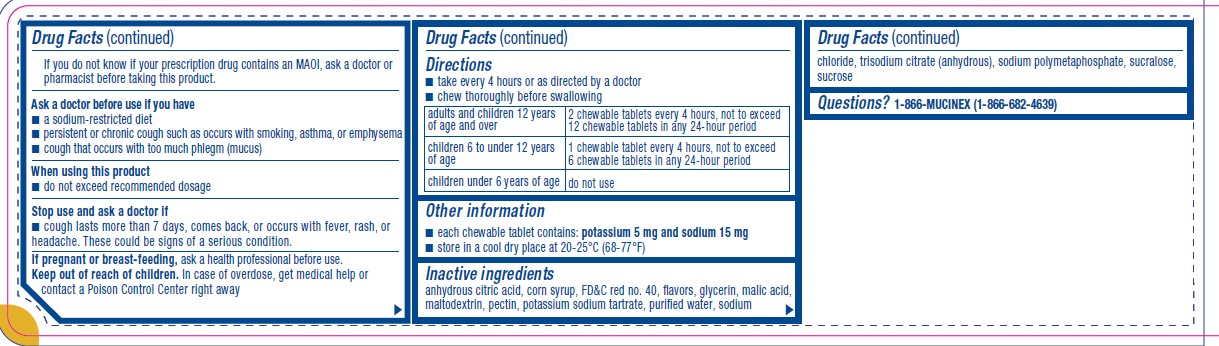
Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.Ask a doctor before use if you have
■ a sodium-restricted diet
■ persistent or chronic cough such as occurs with smoking, asthma, or emphysema
■ cough that occurs with too much phlegm (mucus)When using this product
■ do not exceed recommended dosageStop use and ask a doctor if
■ cough lasts more than 7 days, comes back, or occurs with fever, rash, or
headache. These could be signs of a serious condition. - KEEP OUT OF REACH OF CHILDREN
-
Directions
Directions
■ take every 4 hours or as directed by a doctor
■ chew thoroughly before swallowingadults and children 12 years
of age and overchewable tablets every 4 hours, not to exceed
12 chewable tablets in any 24-hour periodchildren 6 to under 12 years
of age1 chewable tablet every 4 hours, not to exceed
6 chewable tablets in any 24-hour periodchildren under 6 years of age do not use - OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MUCINEX CHILDRENS MIGHTY CHEWS COUGH
dextromethorphan hydrobromide tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72854-081 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg Inactive Ingredients Ingredient Name Strength CORN SYRUP (UNII: 9G5L16BK6N) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) PECTIN (UNII: 89NA02M4RX) POTASSIUM SODIUM TARTRATE (UNII: QH257BPV3J) SUCROSE (UNII: C151H8M554) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ANHYDROUS TRISODIUM CITRATE (UNII: RS7A450LGA) SUCRALOSE (UNII: 96K6UQ3ZD4) MALIC ACID (UNII: 817L1N4CKP) MALTODEXTRIN (UNII: 7CVR7L4A2D) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM POLYMETAPHOSPHATE (UNII: P1BM4ZH95L) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color red Score no score Shape ROUND Size 234mm Flavor BERRY (Mixed Berry) Imprint Code M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72854-081-16 16 in 1 BOTTLE; Type 0: Not a Combination Product 06/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/03/2024 Labeler - RB Health (US) LLC (081049410)