Label: TULIP DEW SHIELD GLOW- zinc oxide stick
- NDC Code(s): 82548-2420-1
- Packager: Bloom effects, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTActive Ingredients - Zinc Oxide 14.7%
-
WHEN USINGWhen using this product keep out of eyes. Rinse with water to remove.
-
WARNINGSFor external use only.
-
STOP USEStop use & ask a doctor if skin eash occurs.
-
KEEP OUT OF REACH OF CHILDRENKeep out of reach of children. If swallowed, get medical help or contact a Poison Control Center righ away.
-
DO NOT USEDo not use on damaged or broken skin.
-
INACTIVE INGREDIENTInactive Ingredients - Caprylic/Capric Triglyceride - Butyloctyl Salicylate - Butyrospermum Parkii(Shea) Oil - C12-15 Alkyl Benzoate - Dibutyl Ethylhexanoyl Glutamide - Dibutyl Lauroyl ...
-
PURPOSESunscreen
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
-
PRINCIPAL DISPLAY PANEL
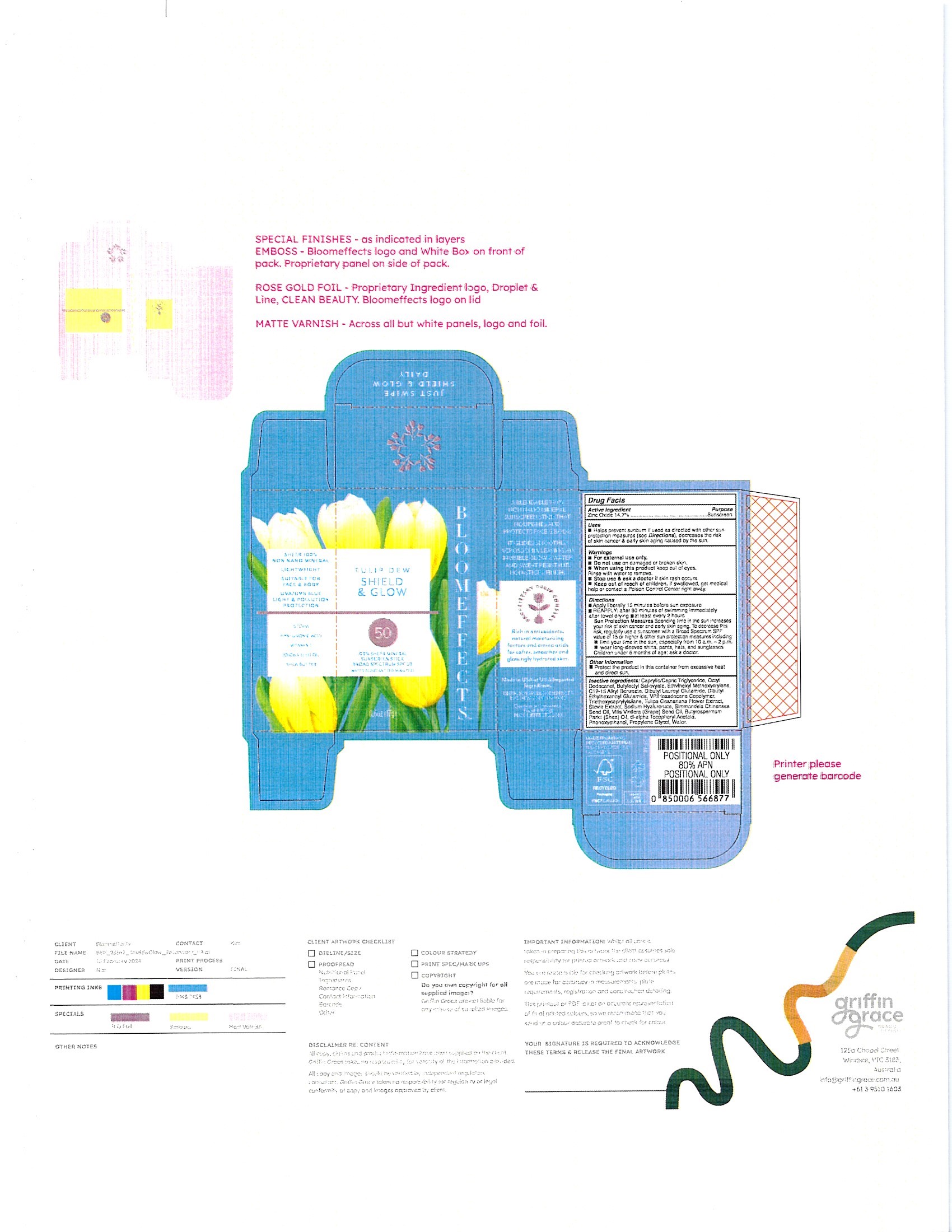
-
INGREDIENTS AND APPEARANCEProduct Information

