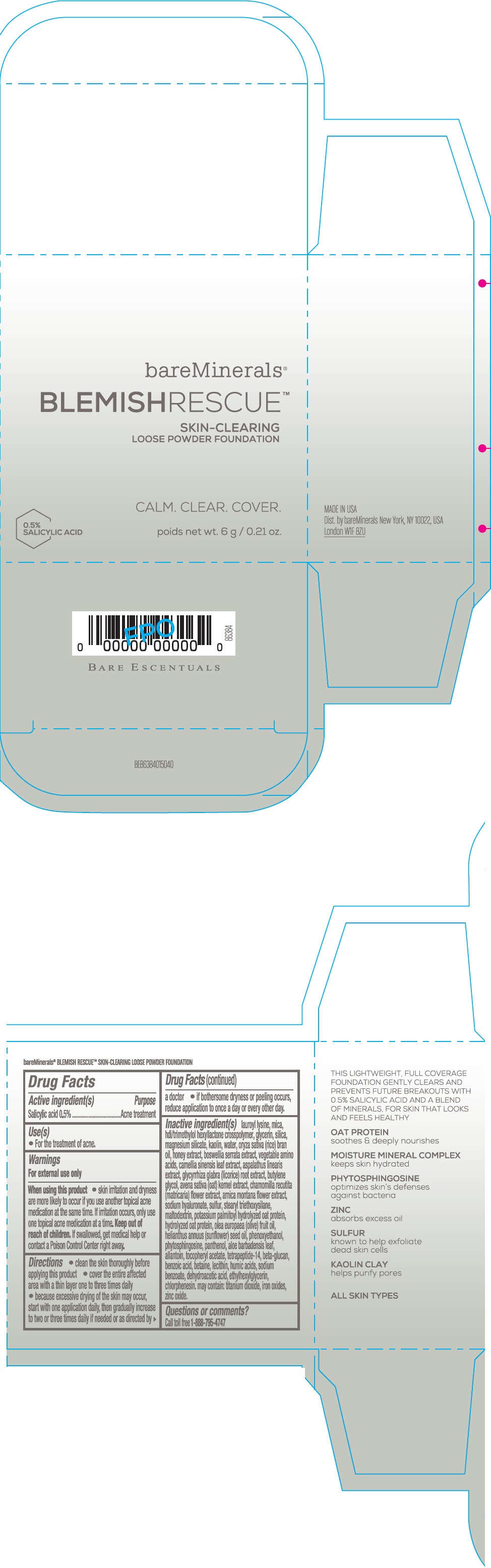
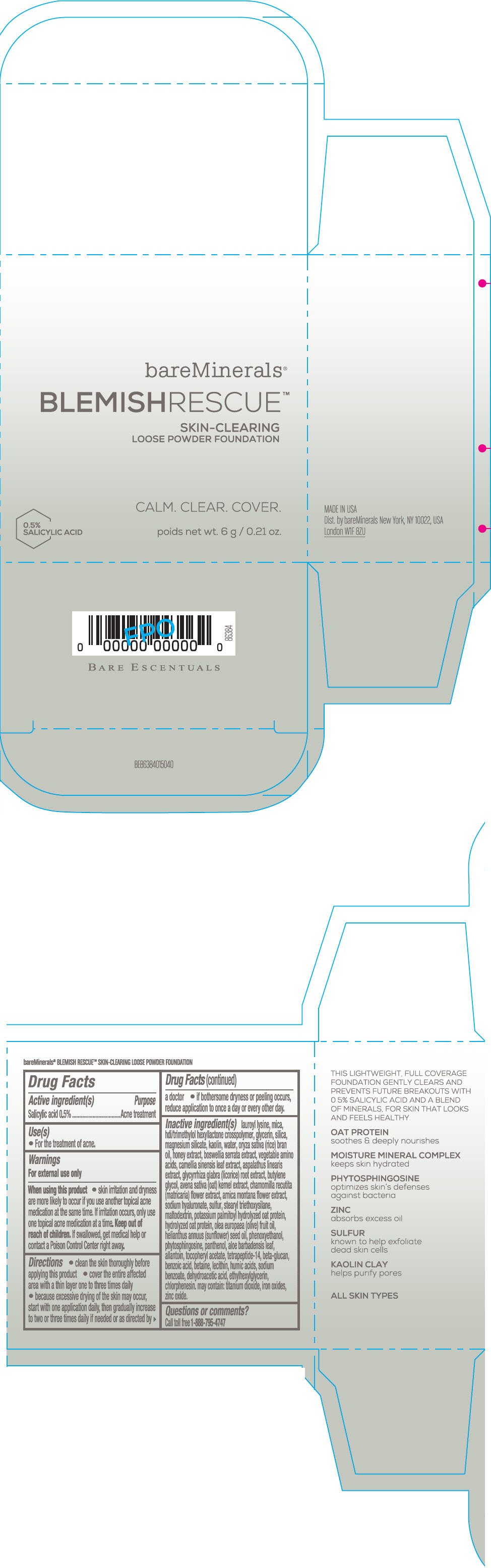
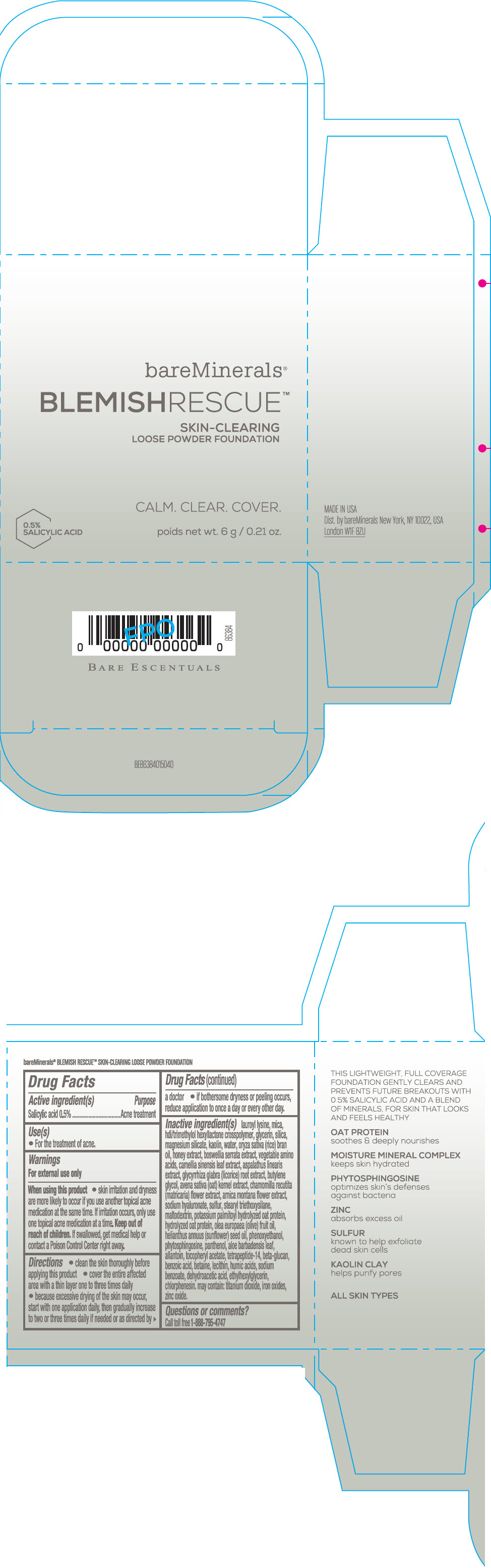
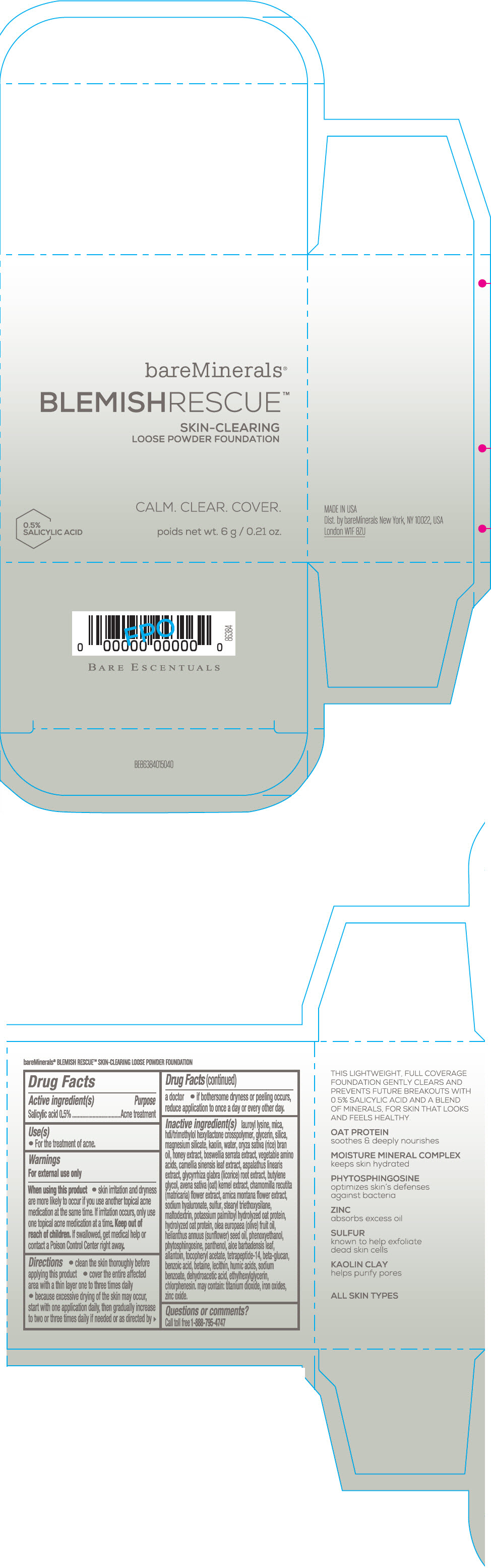
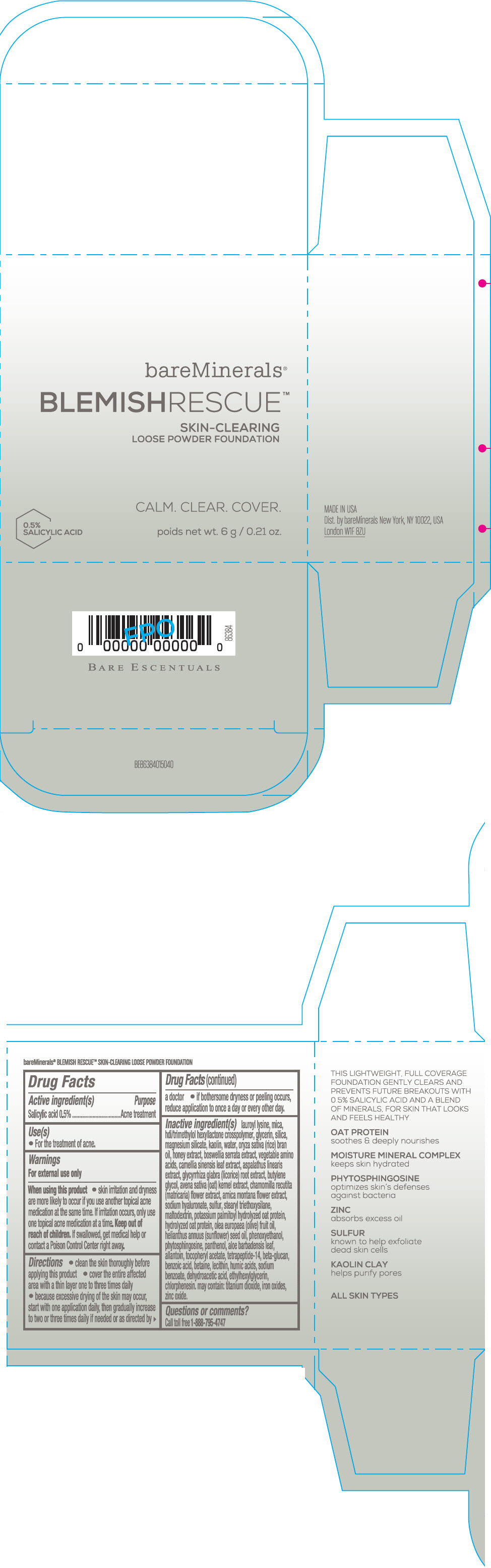
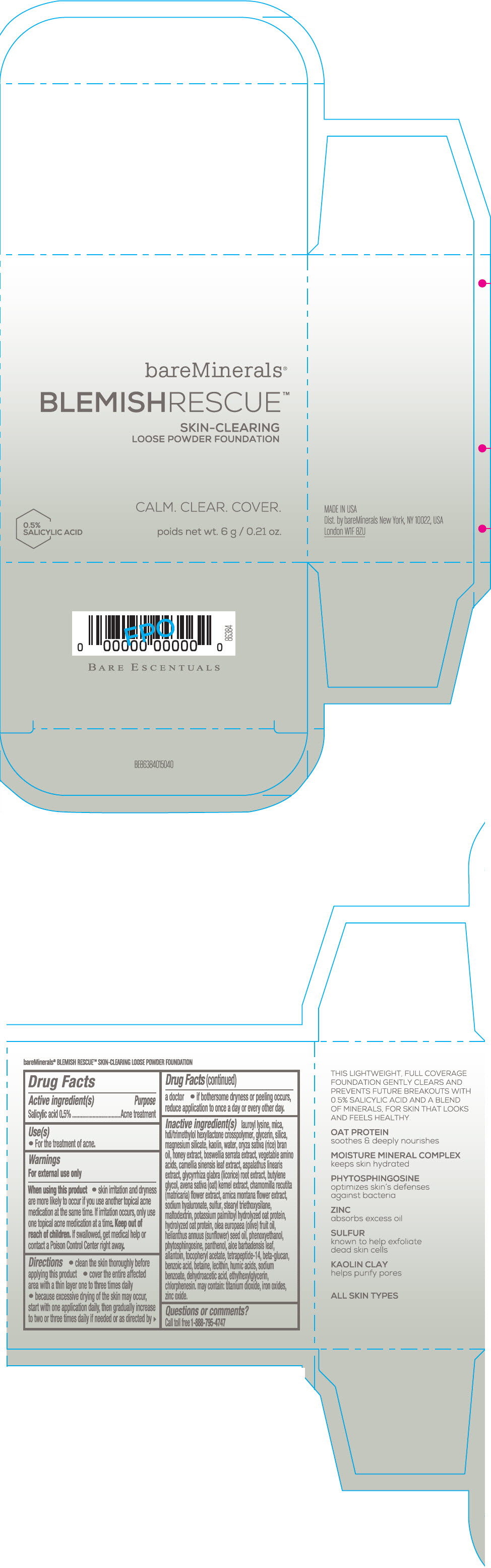
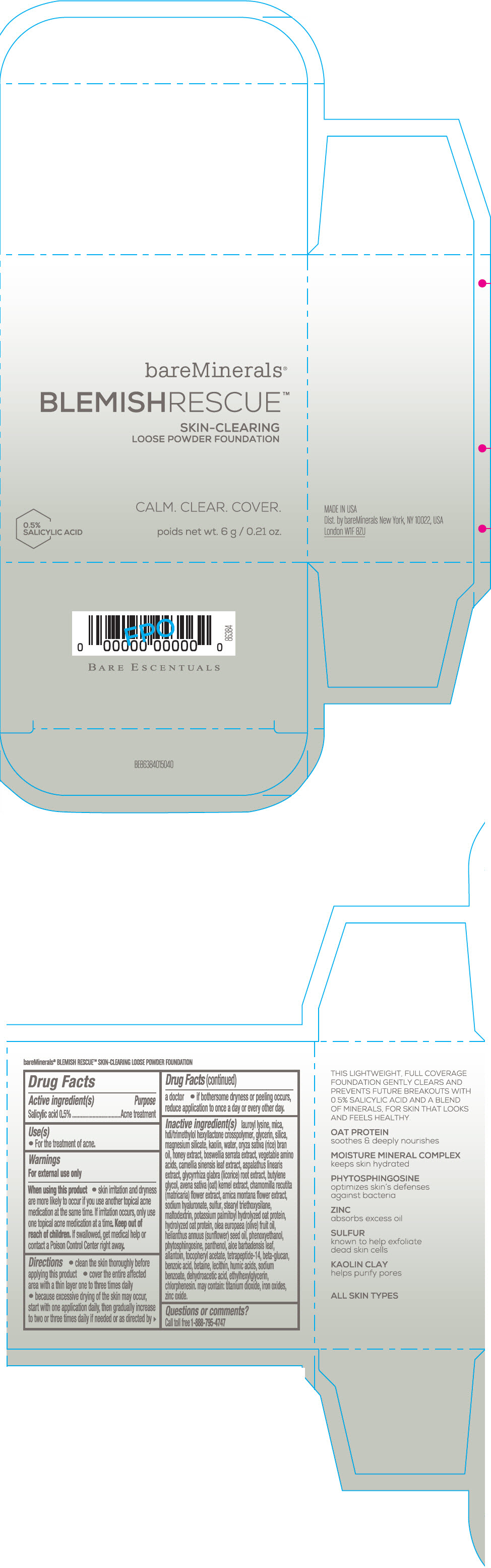
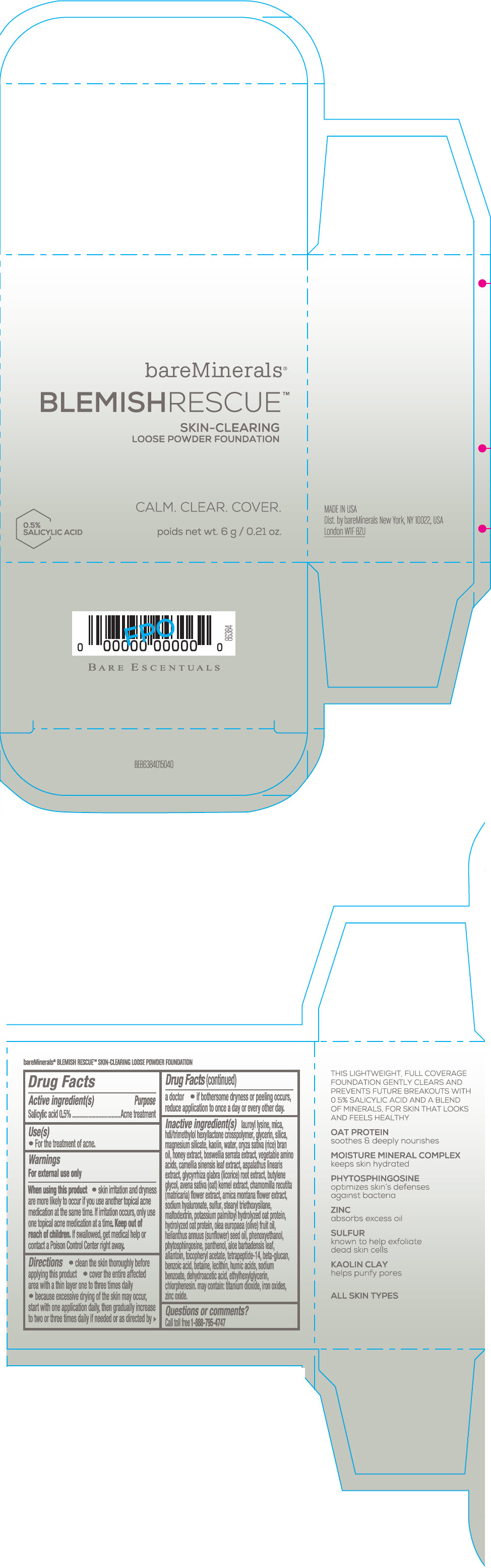
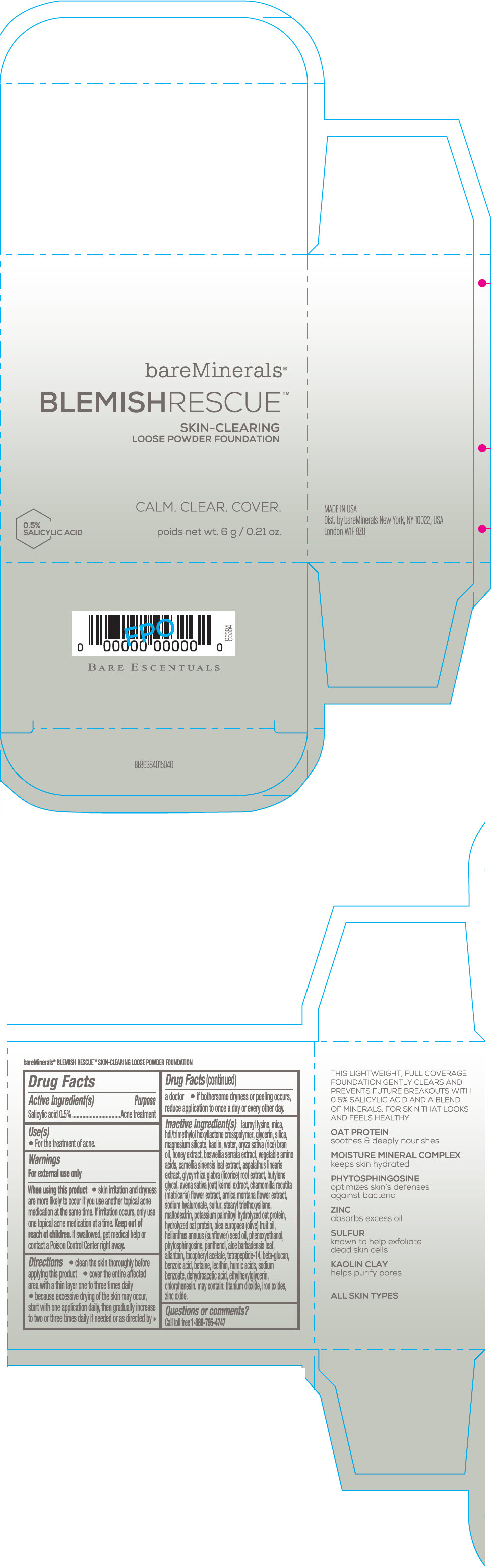
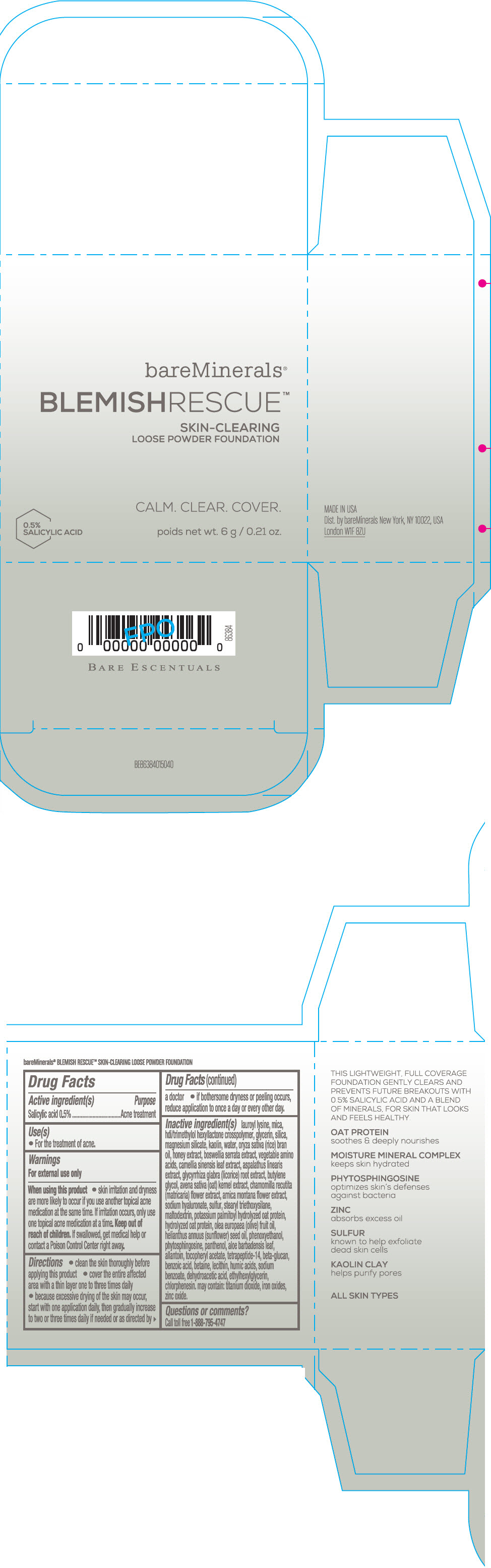
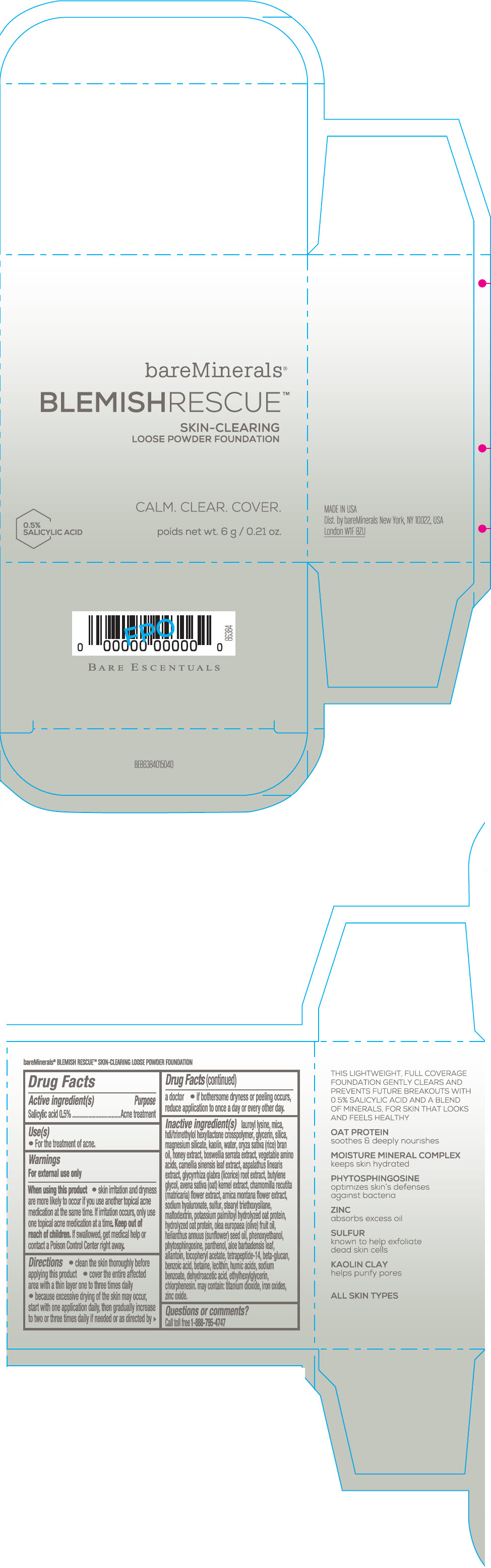
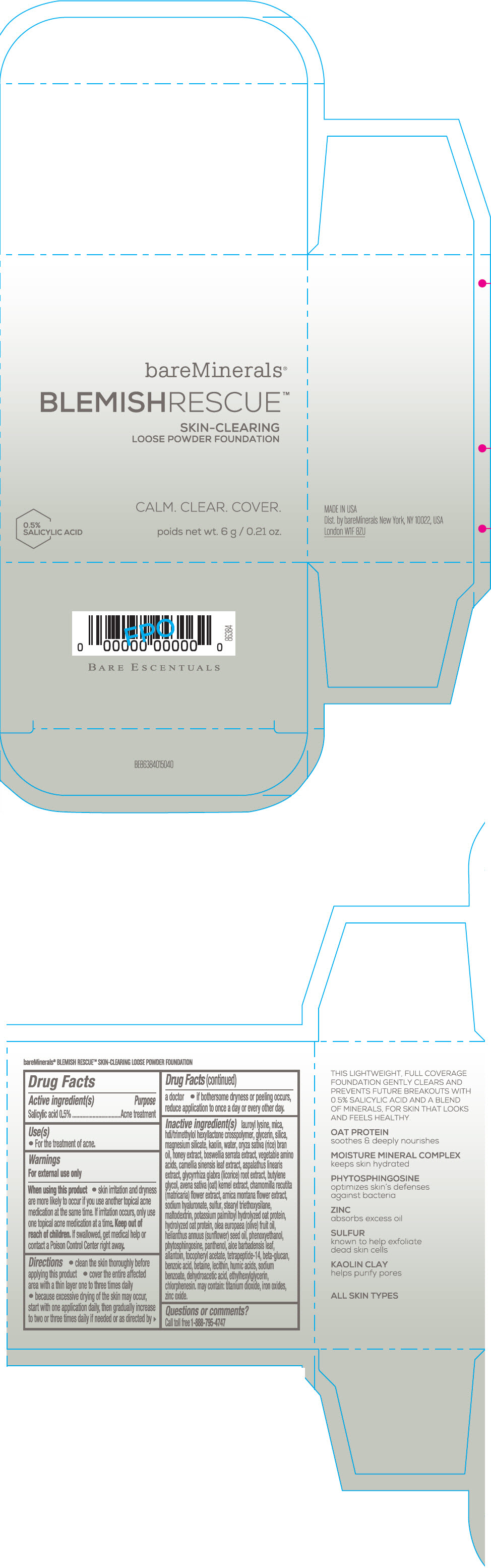
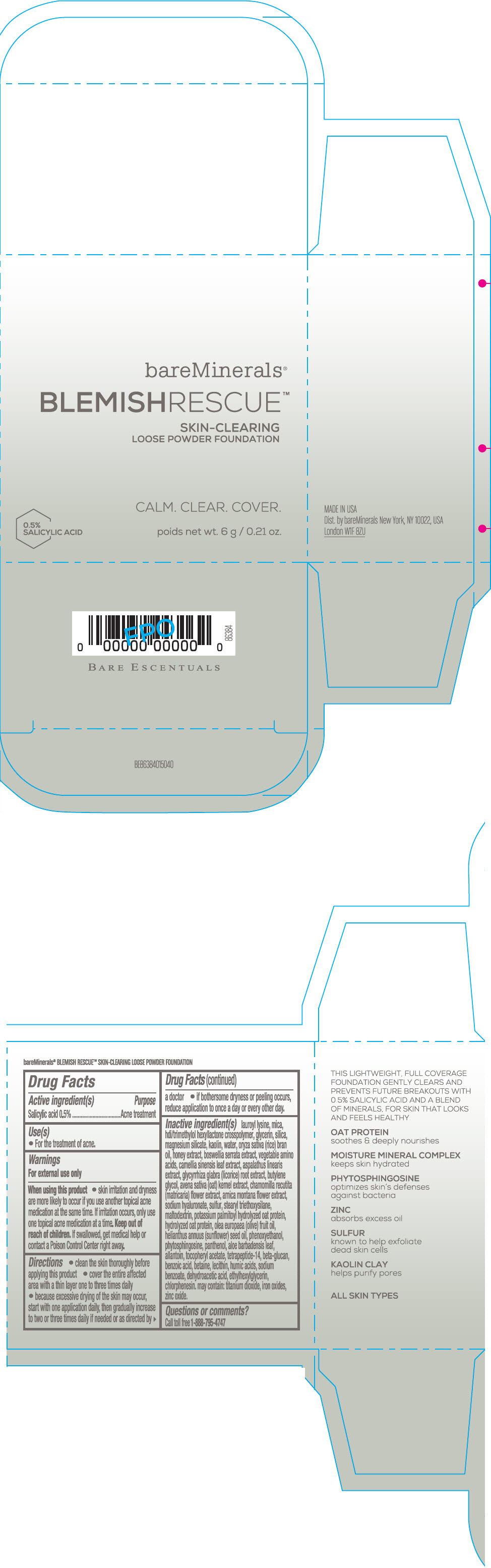
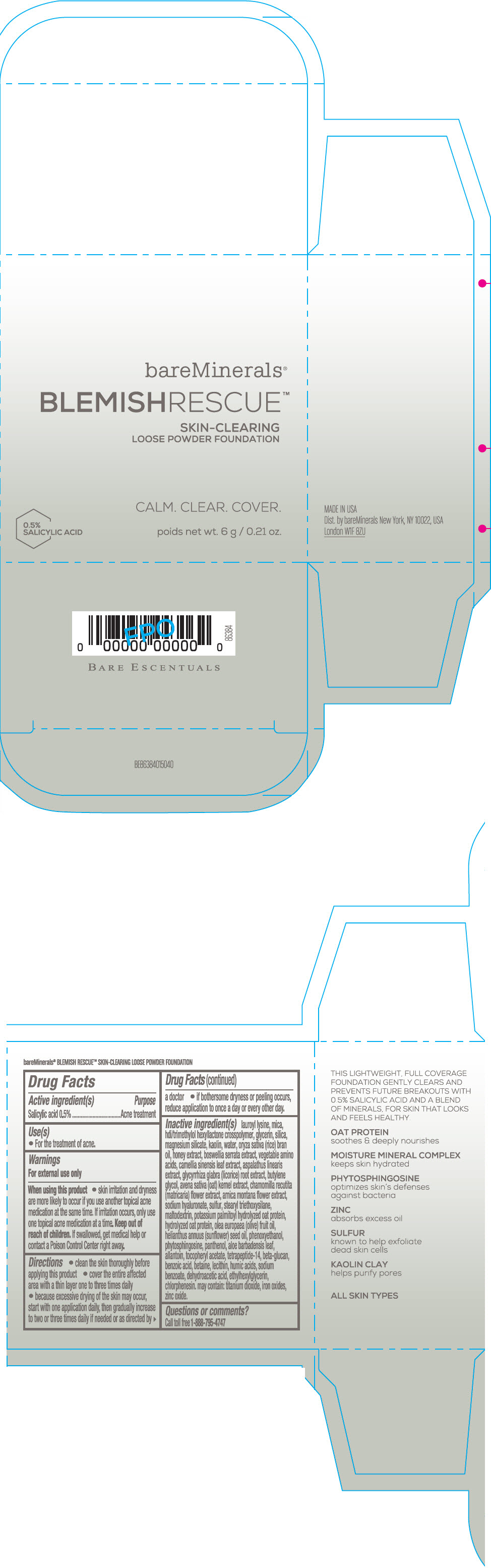
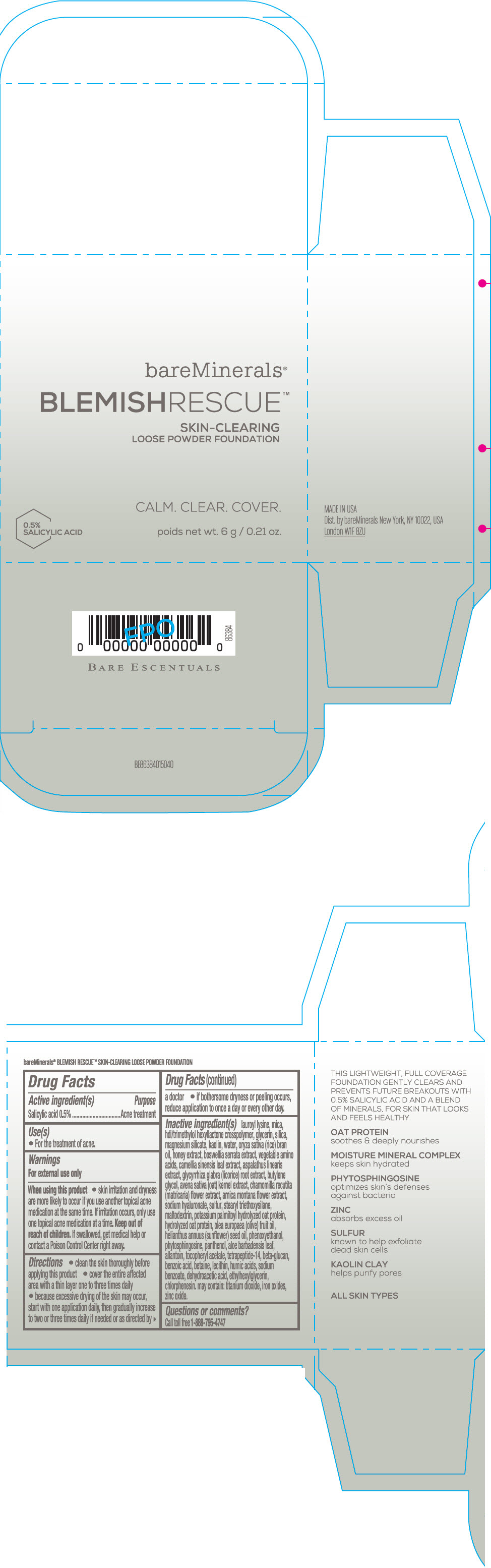
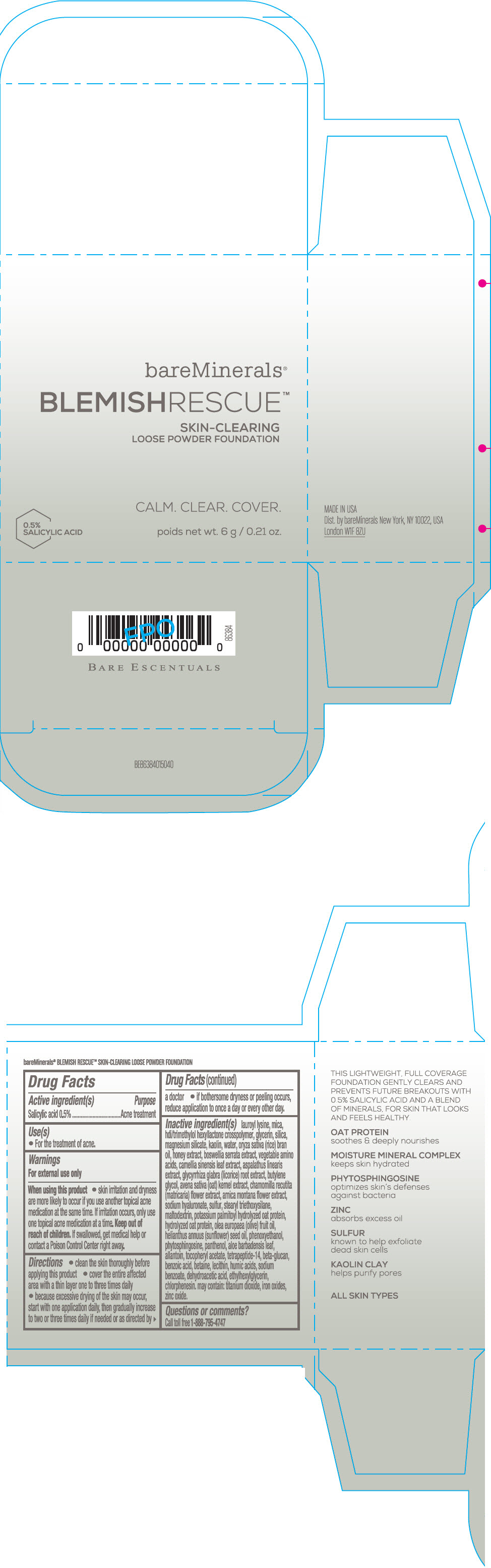
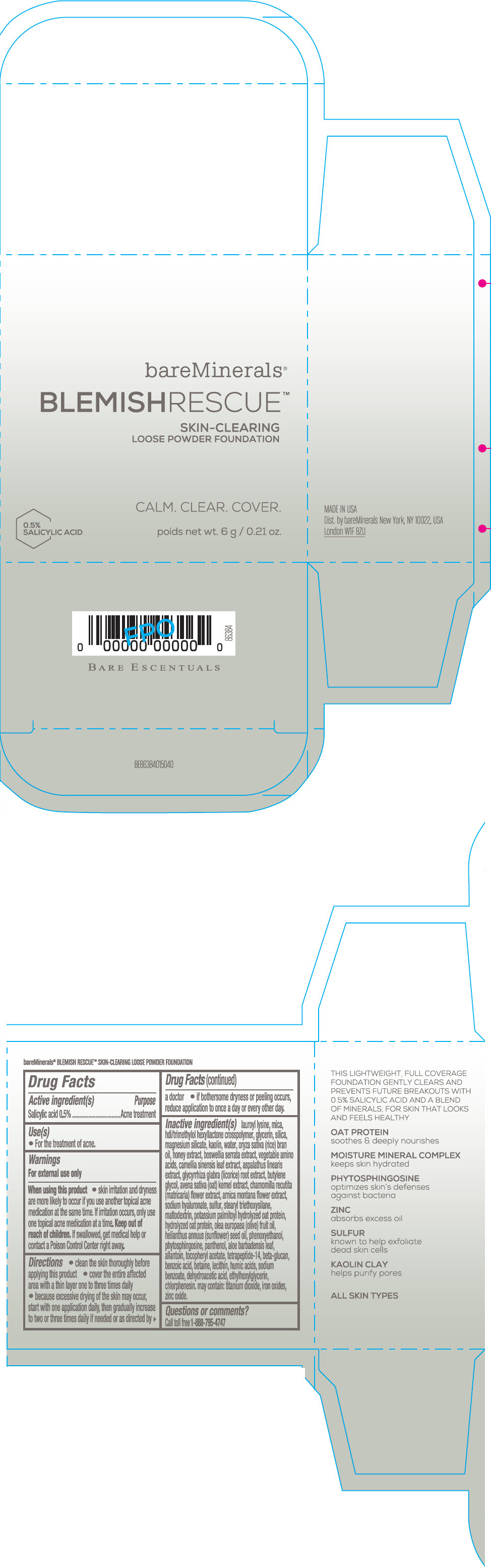
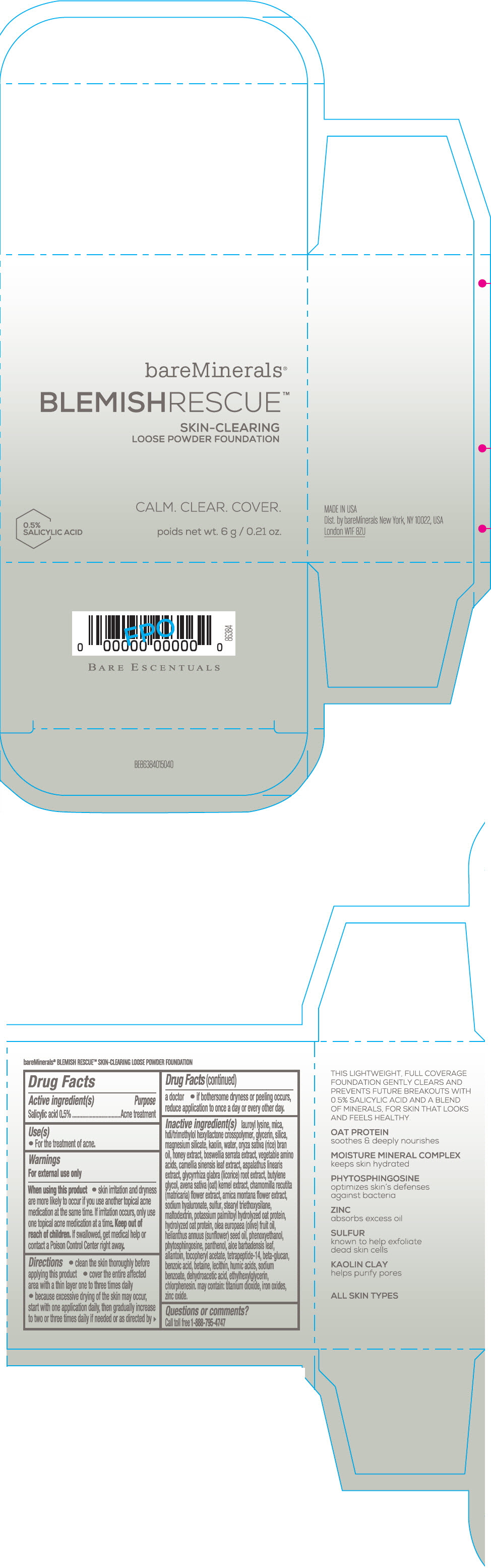
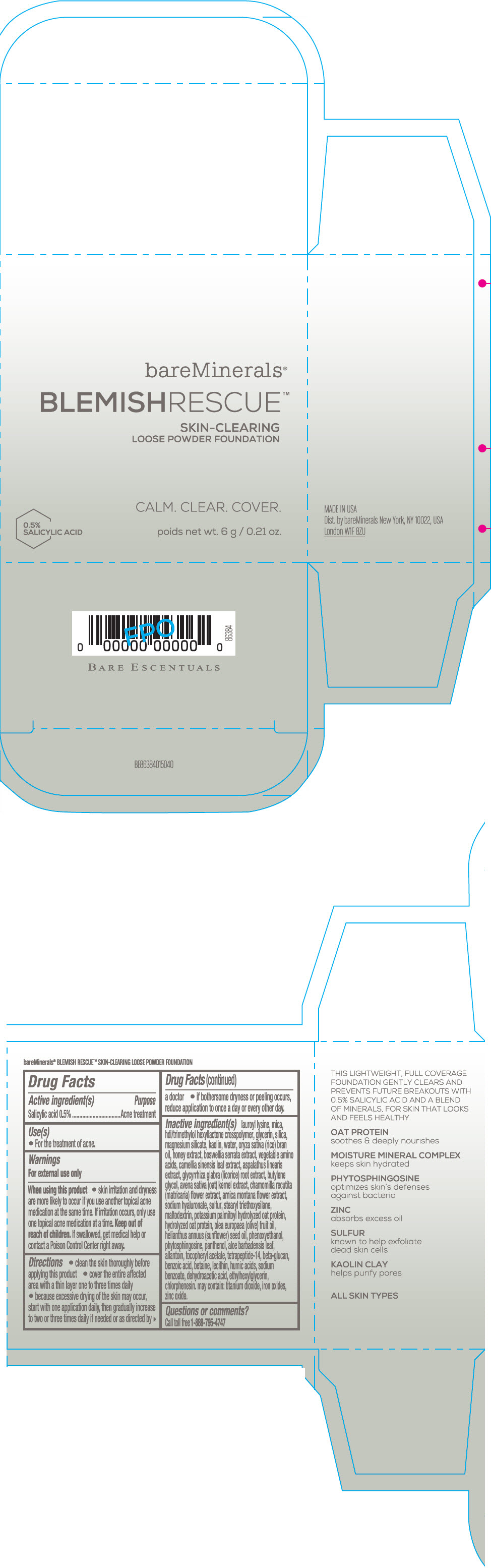
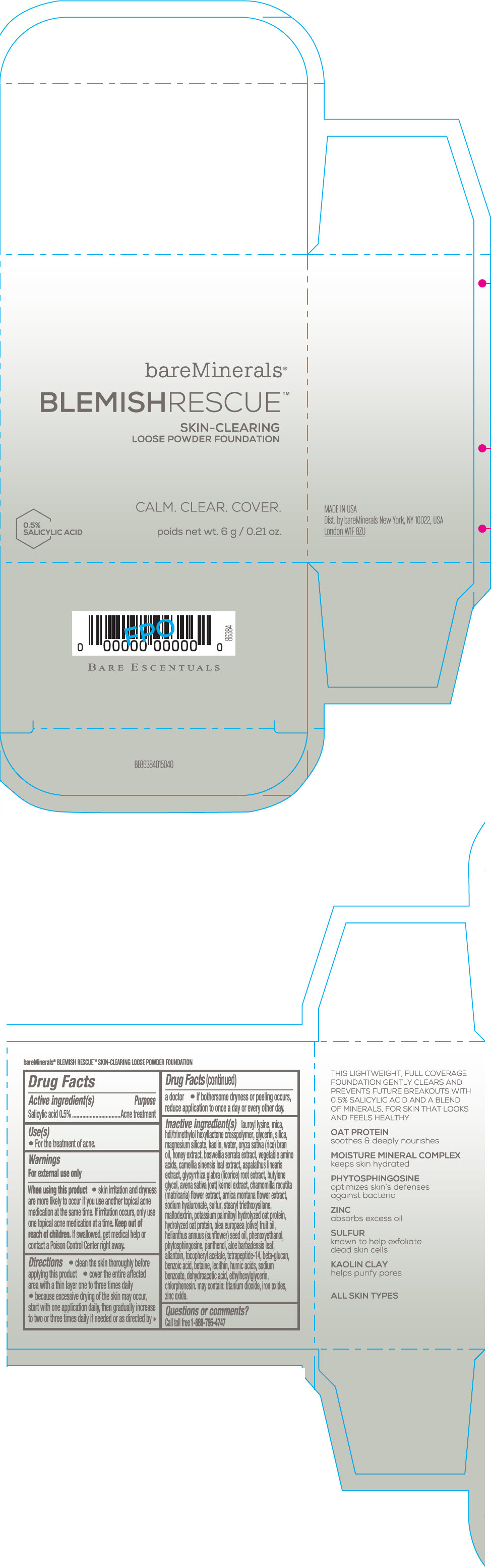
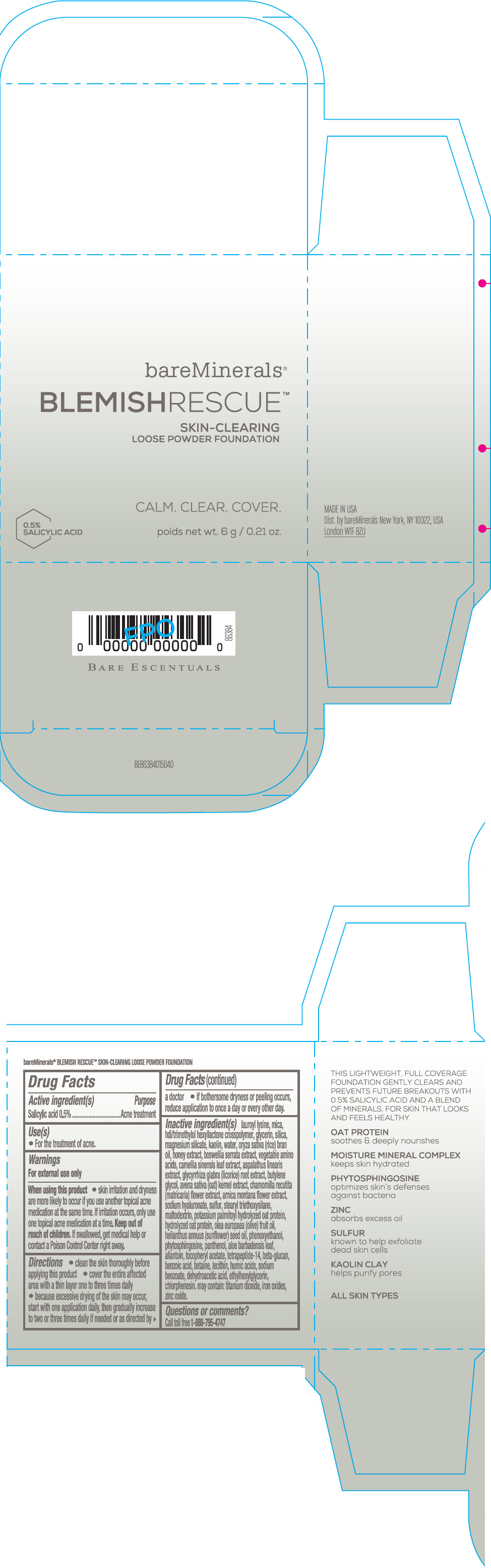
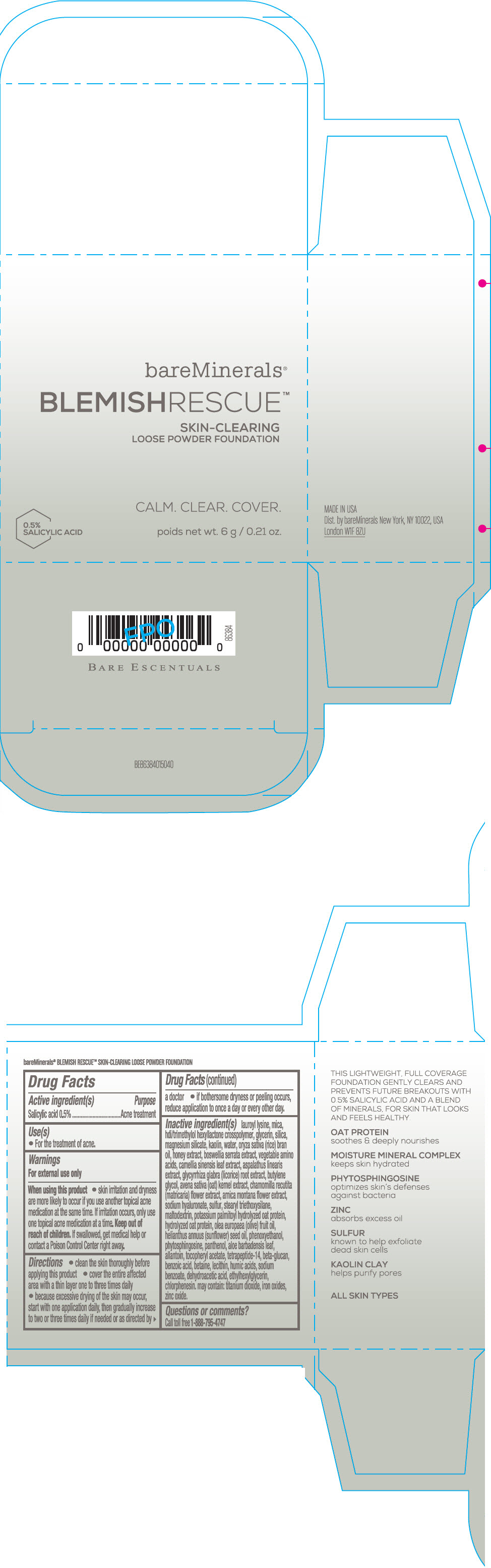
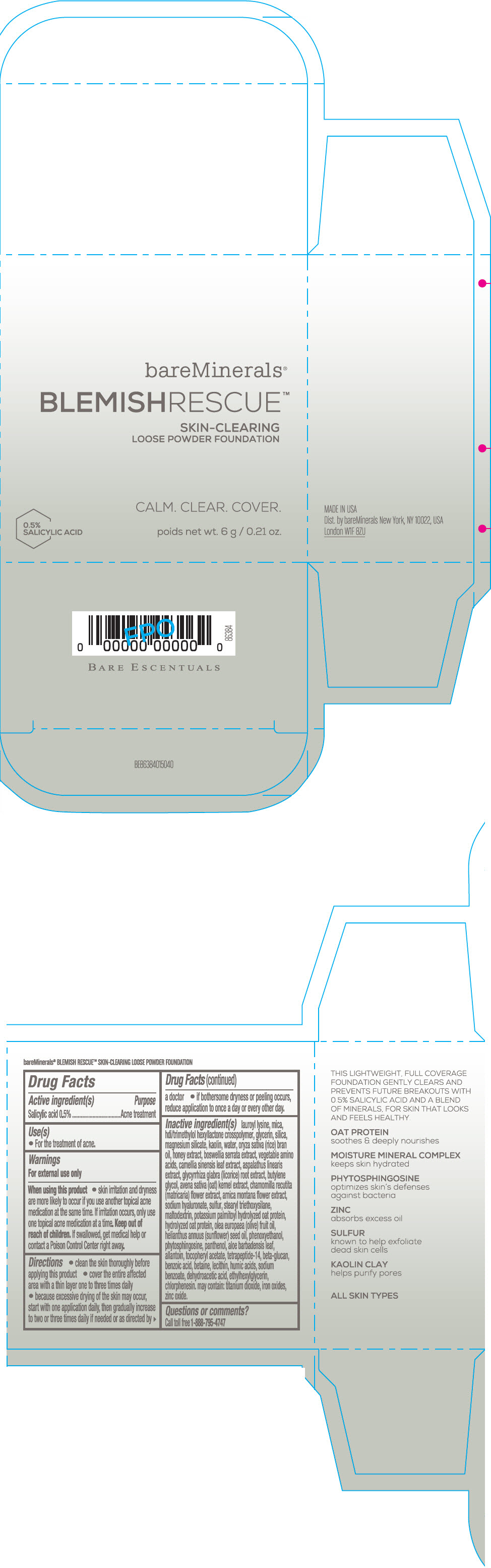
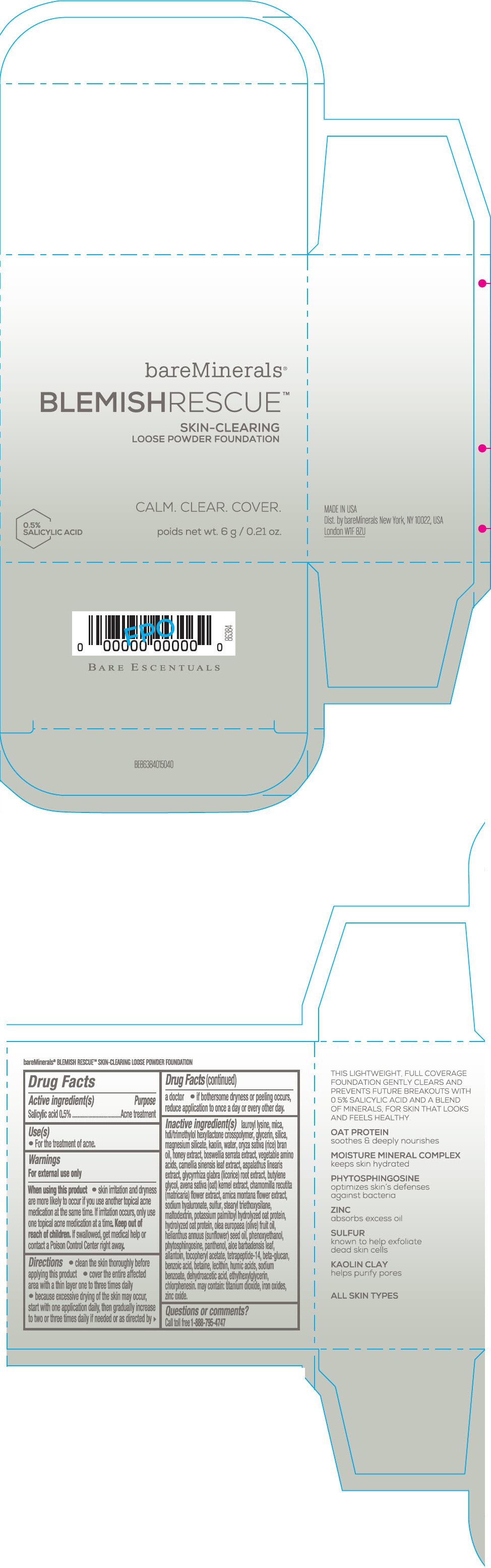
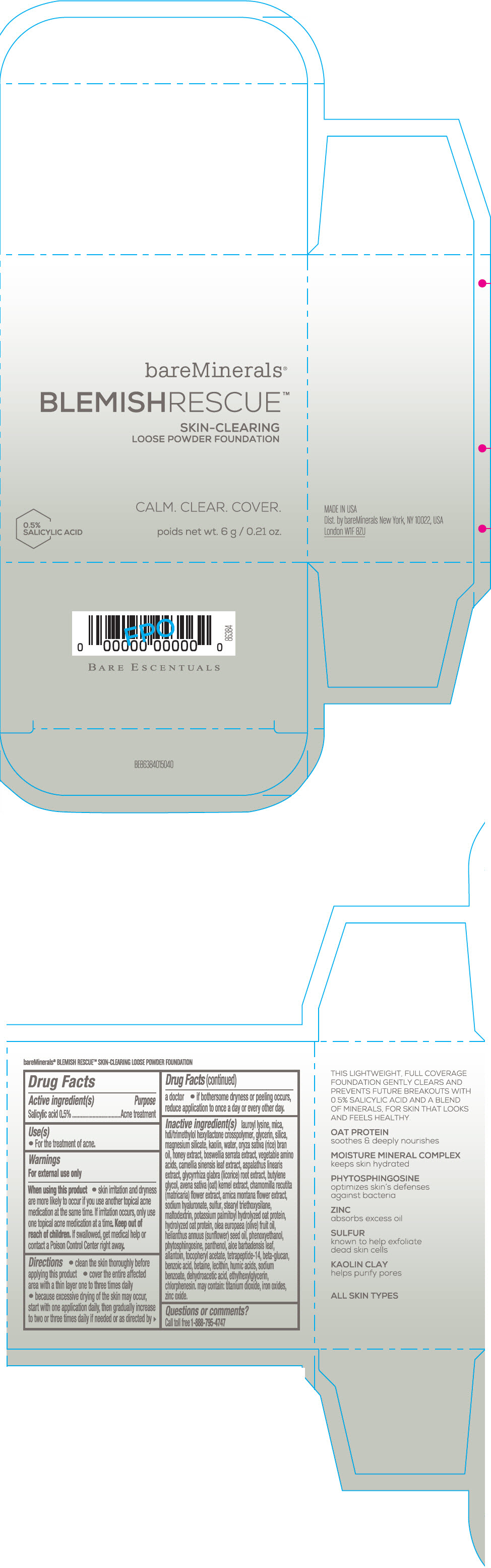
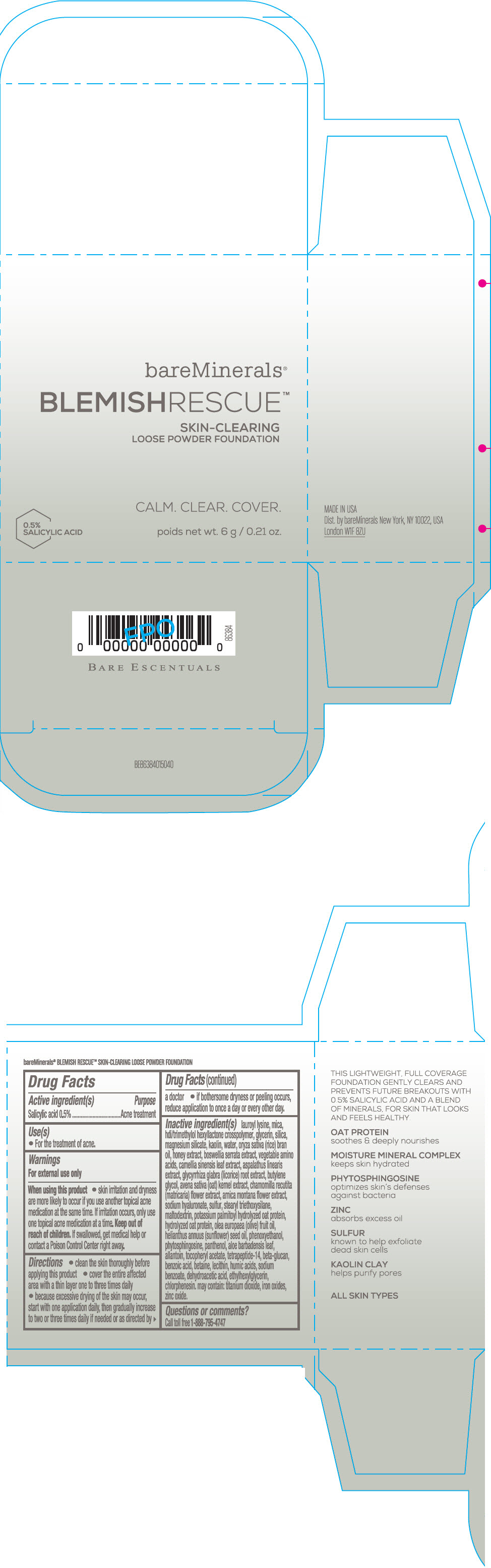
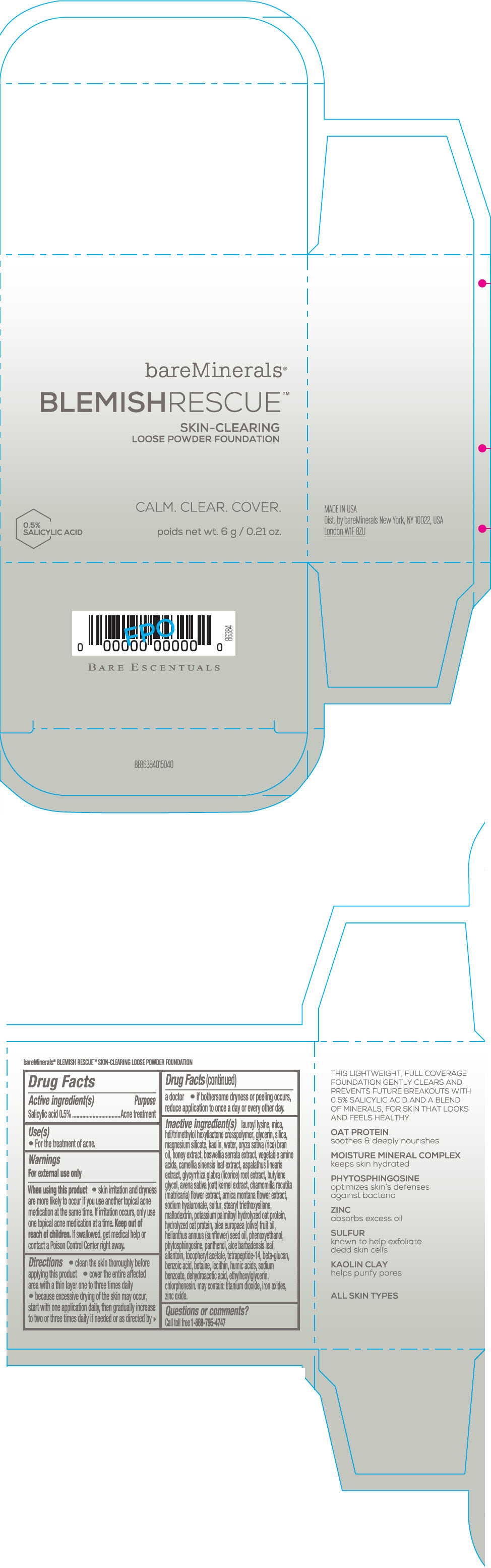
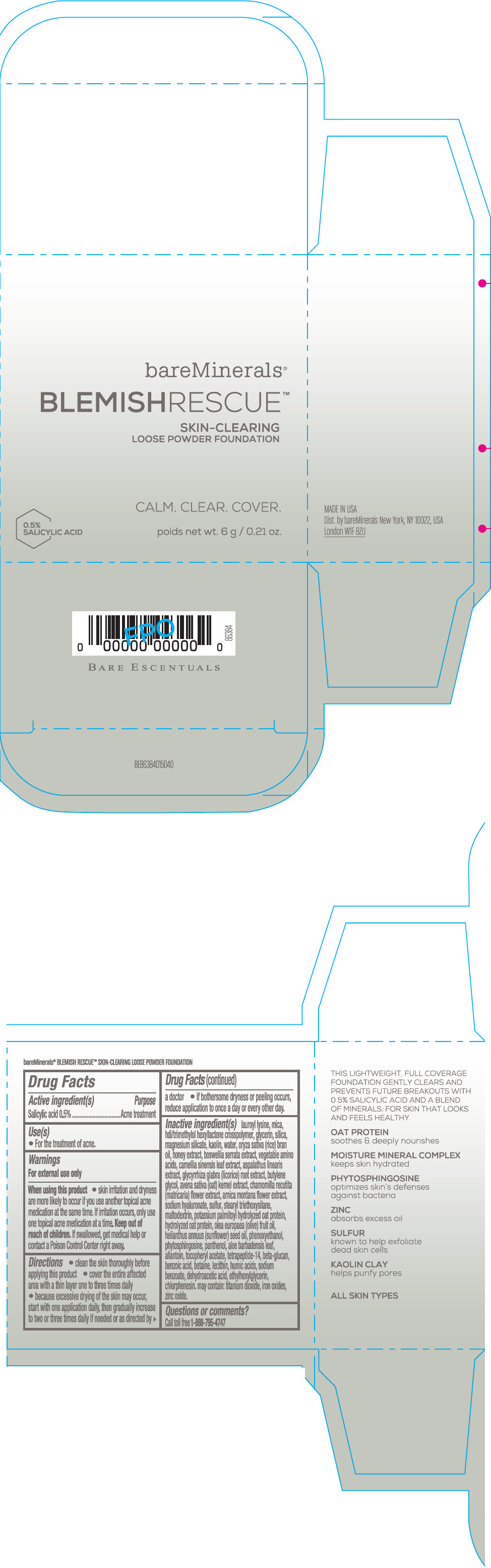
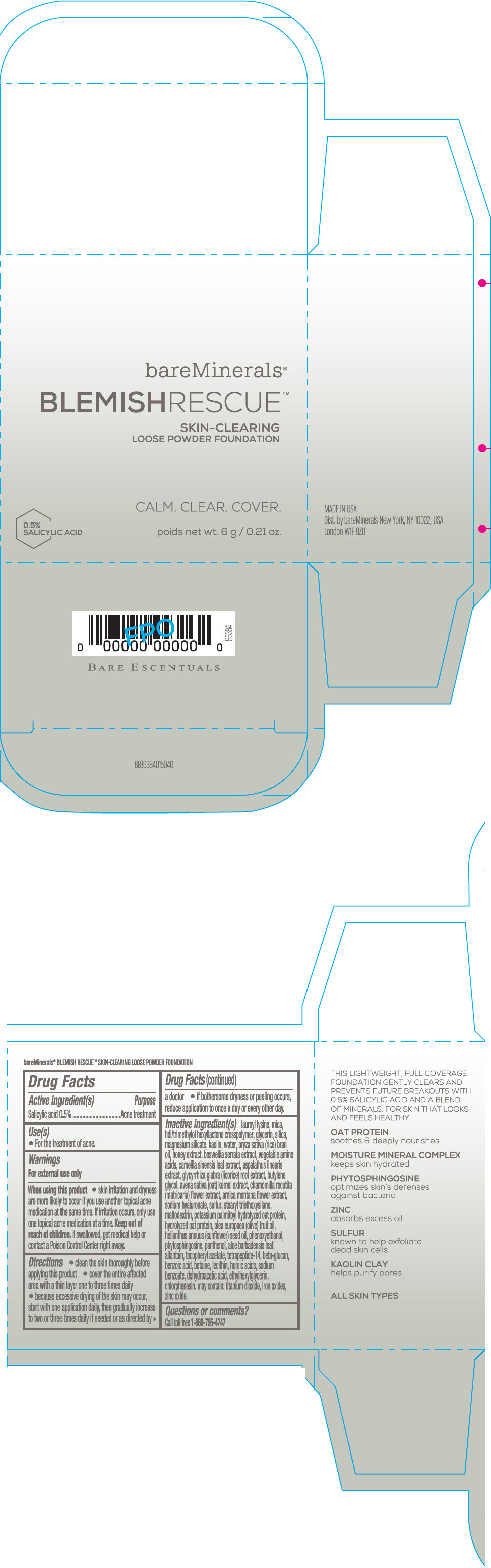
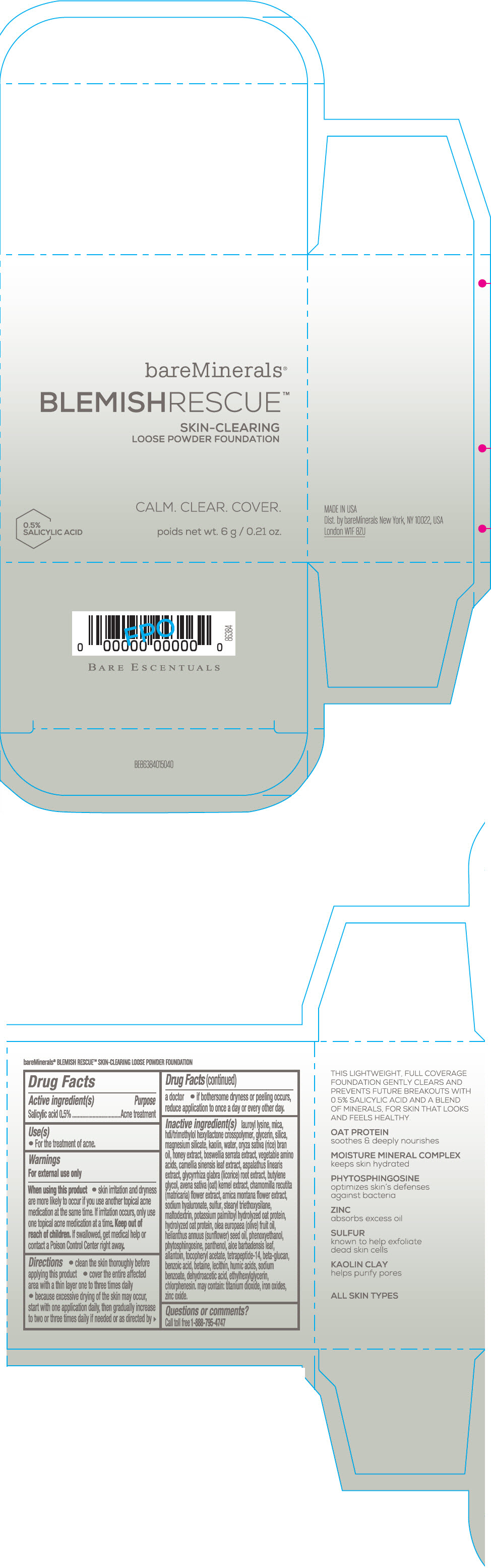
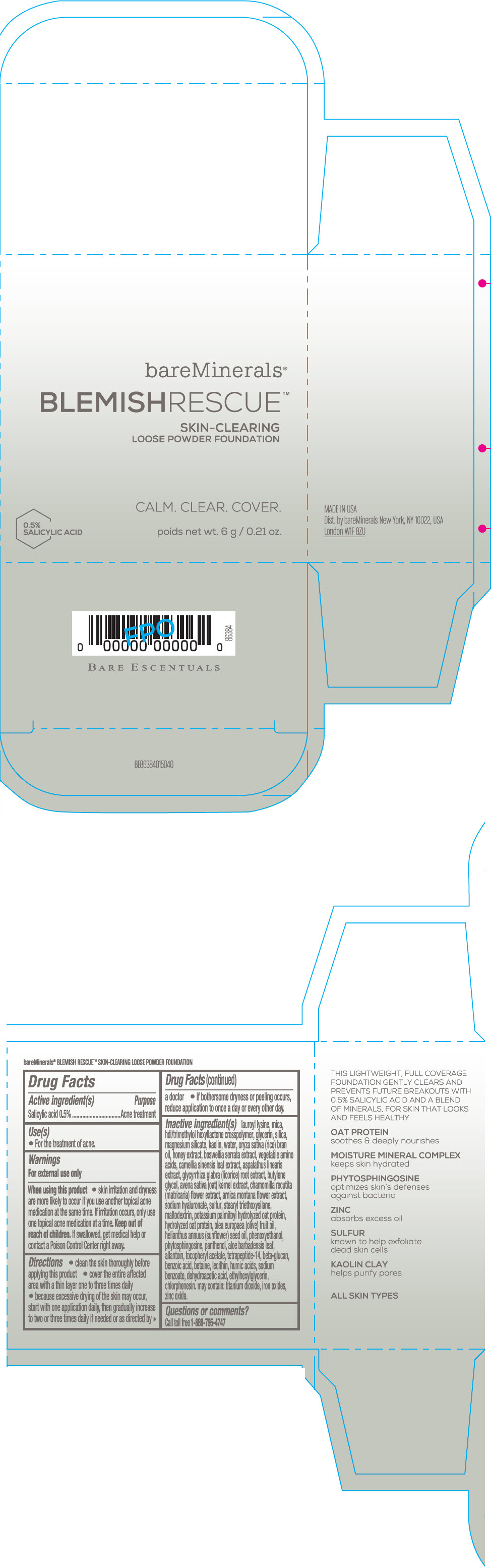
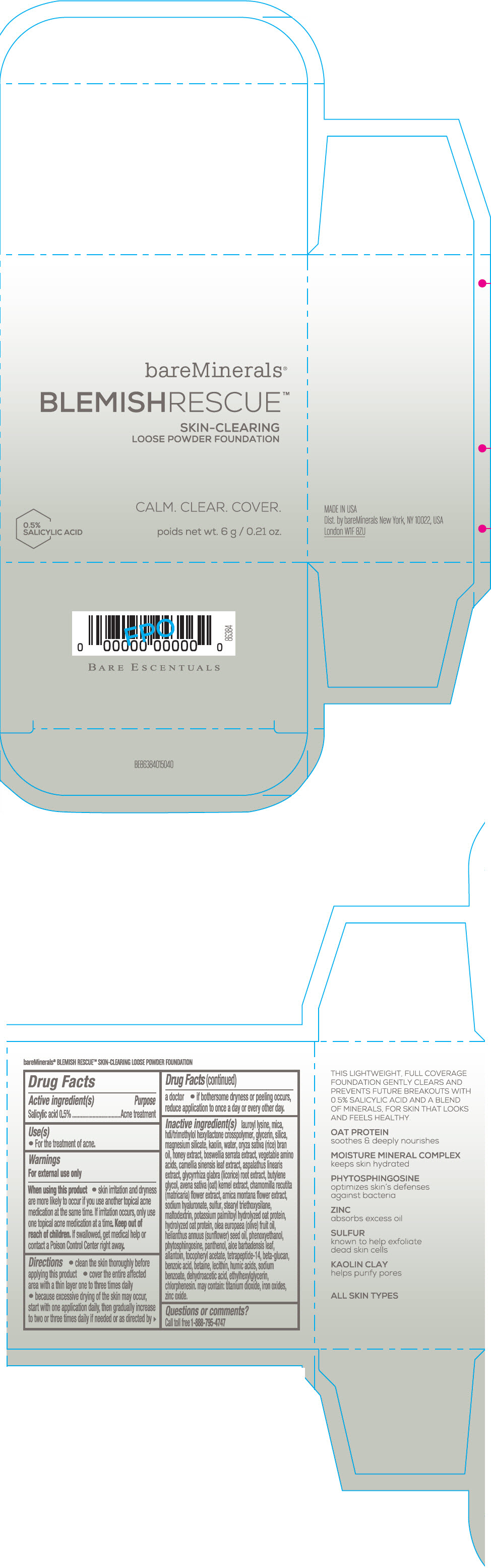
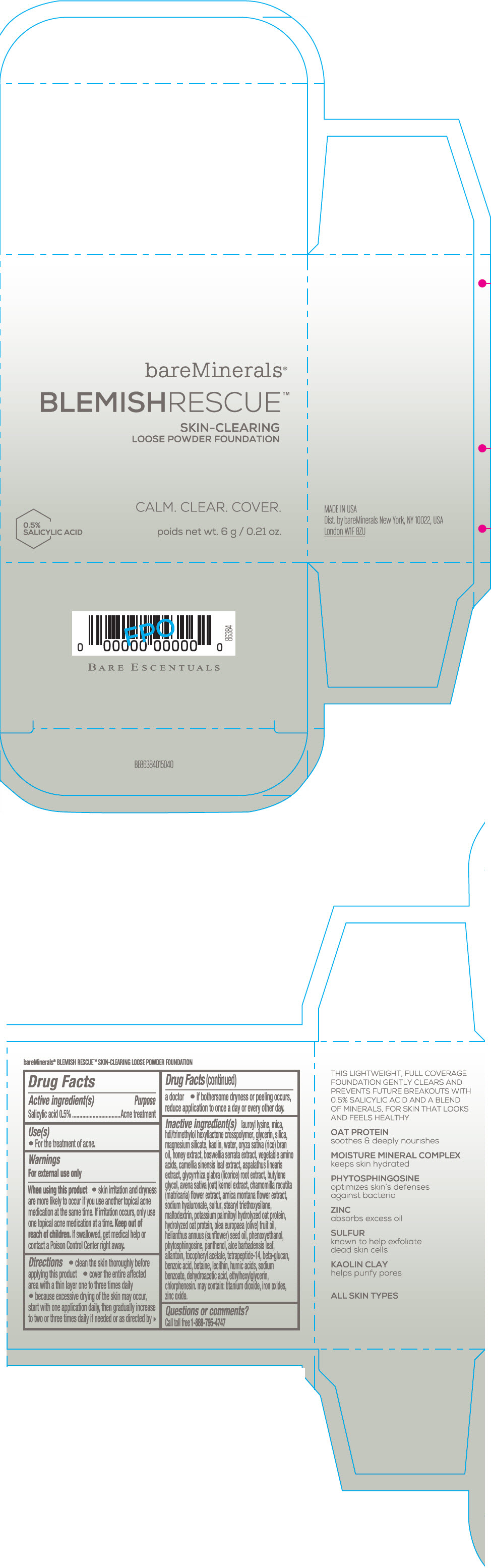
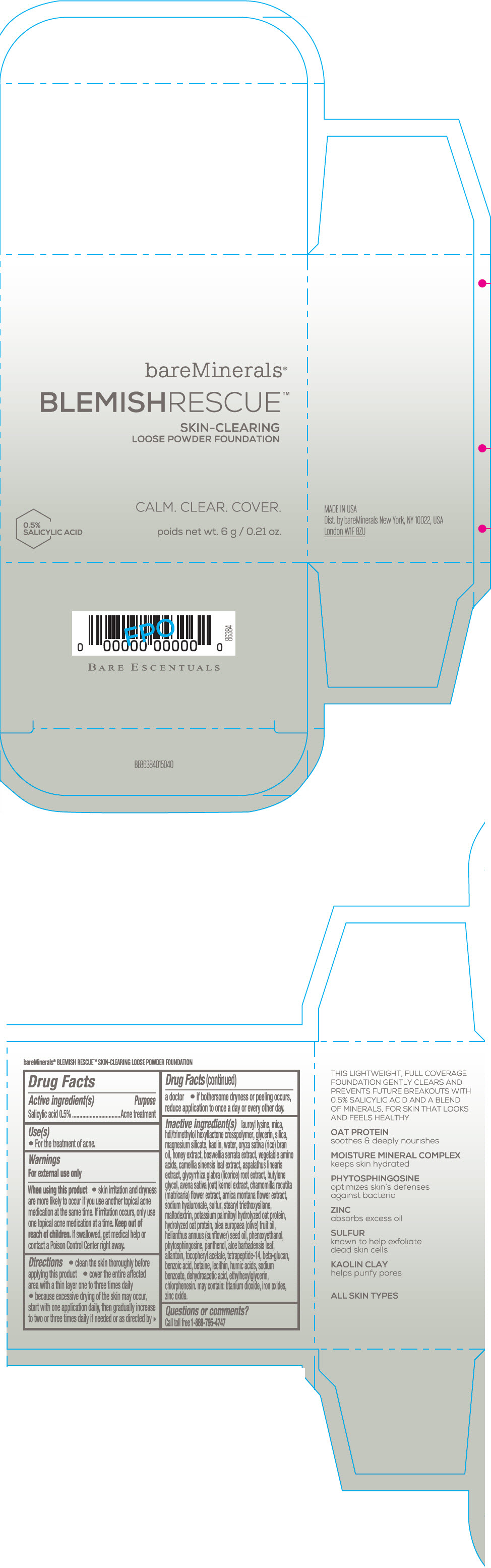
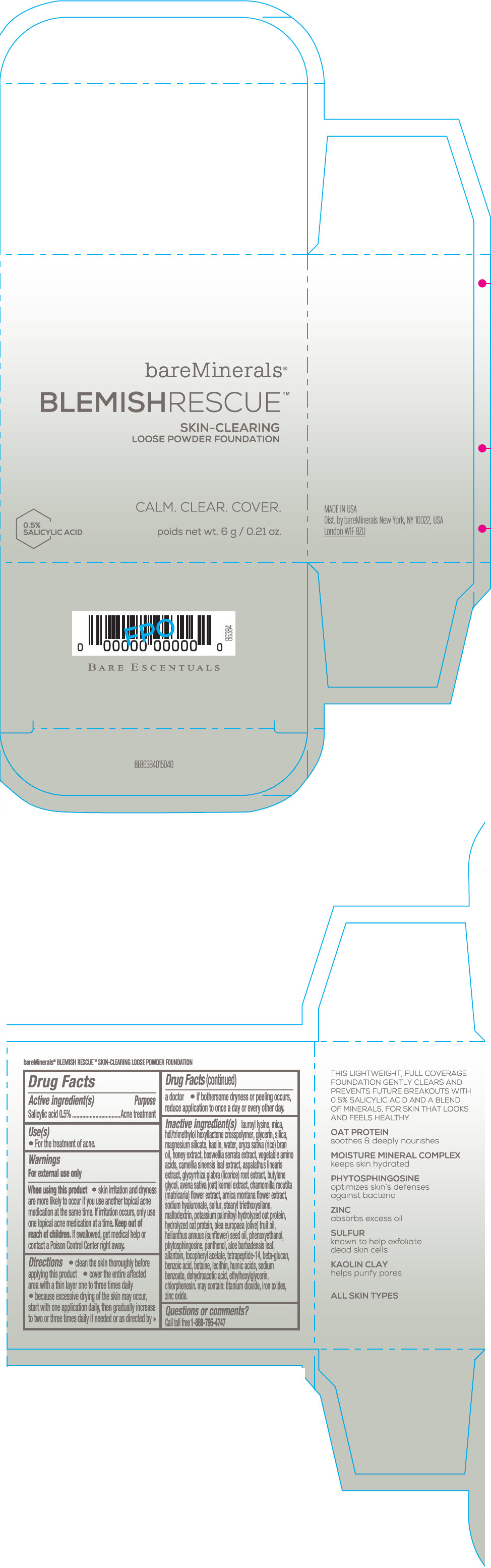
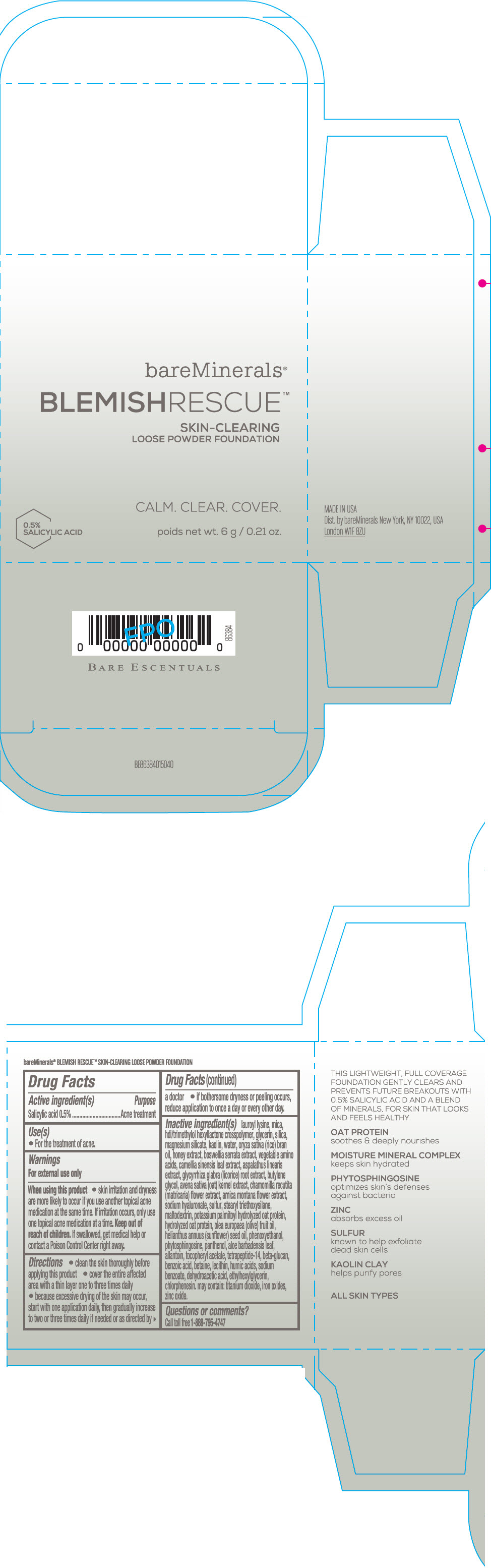
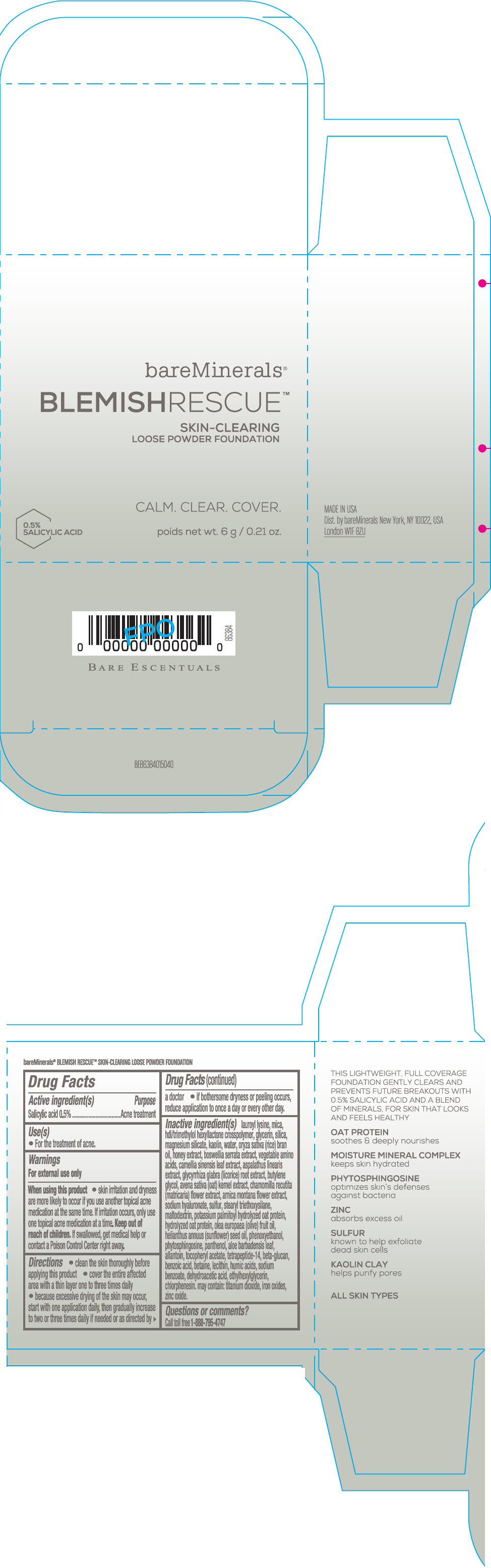
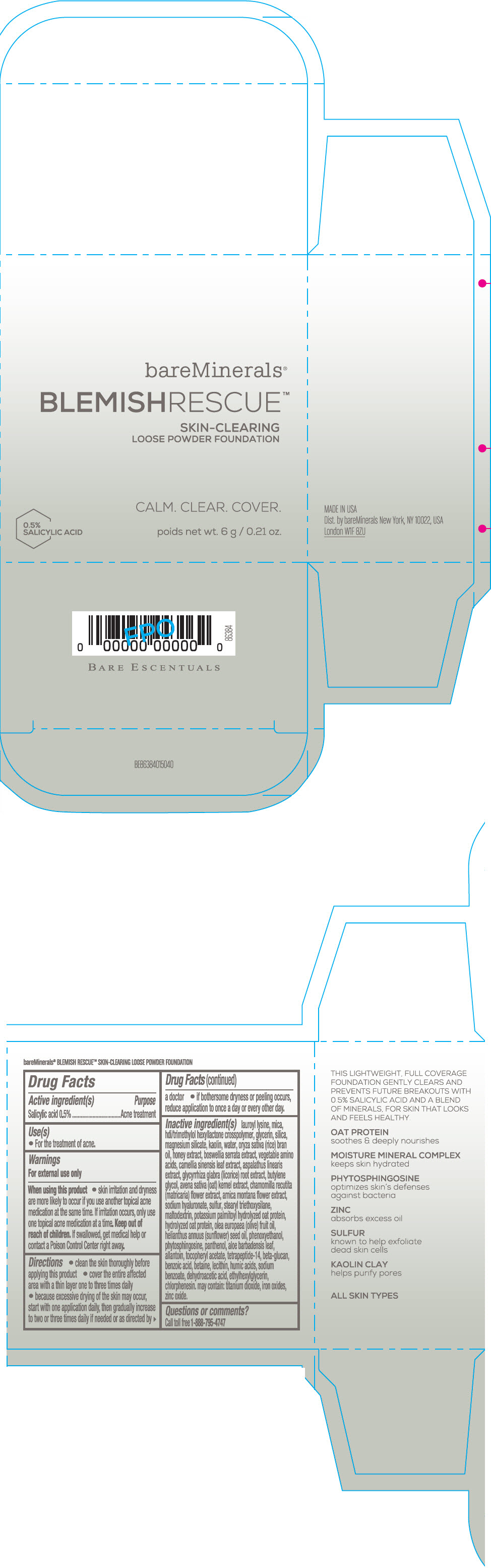
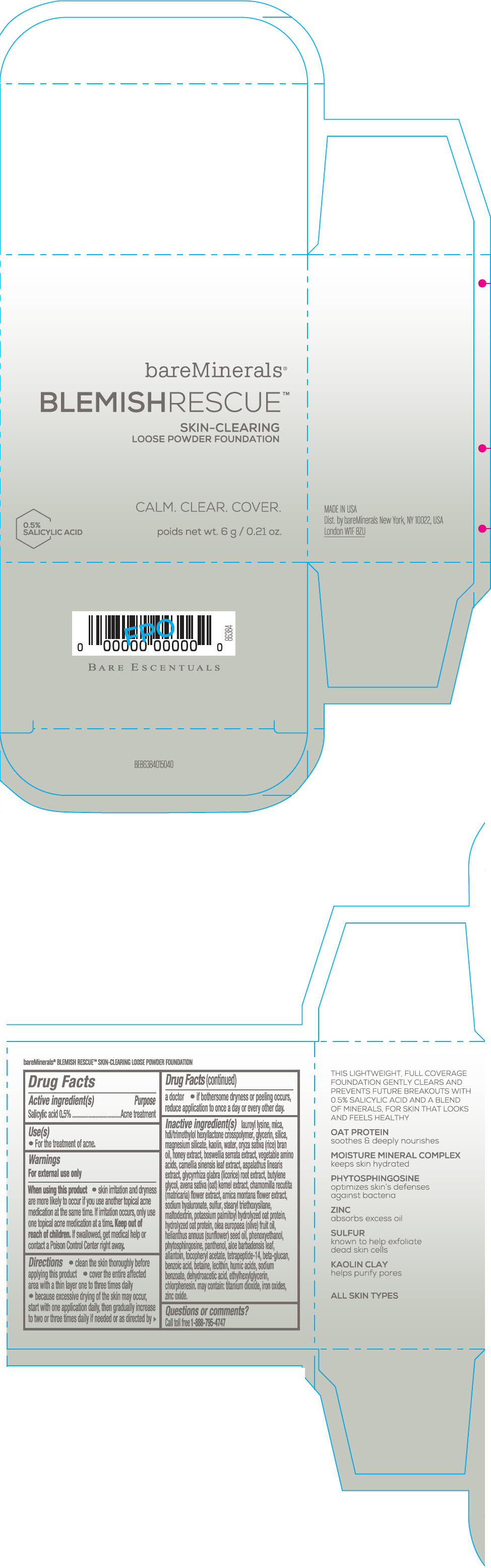
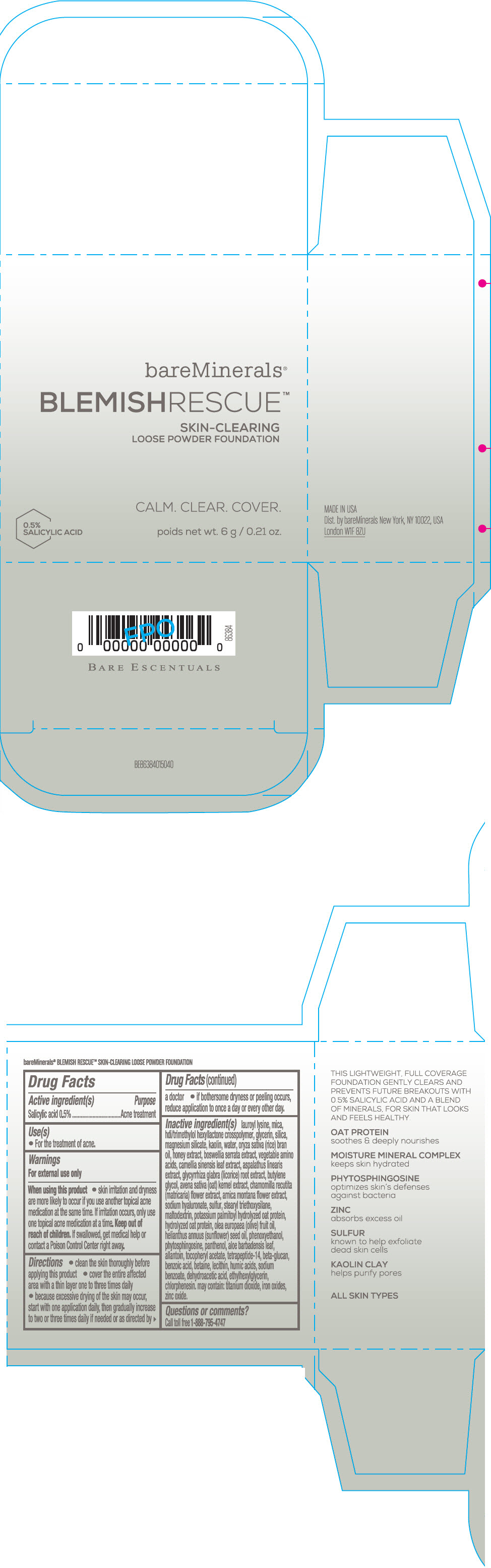
Label: BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION FAIR 1C- salicylic acid powder
BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION FAIR IVORY 1N- salicylic acid powder
BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION FAIRLY LIGHT 1 NW- salicylic acid powder
BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION FAIR .......G LOOSE FOUNDATION MEDIUM DARK 5CN- salicylic acid powder
BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION WARM DEEP 5.5N- salicylic acid powder
BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION NEUTRAL DEEP 5.5NW- salicylic acid powder
BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION DEEPEST DEEP 6C- salicylic acid powder
-
NDC Code(s):
98132-231-03,
98132-231-21,
98132-232-03,
98132-232-21, view more98132-233-03, 98132-233-21, 98132-234-03, 98132-234-21, 98132-235-03, 98132-235-21, 98132-236-03, 98132-236-21, 98132-237-03, 98132-237-21, 98132-238-03, 98132-238-21, 98132-239-03, 98132-239-21, 98132-240-03, 98132-240-21, 98132-241-03, 98132-241-21, 98132-242-03, 98132-242-21, 98132-243-03, 98132-243-21, 98132-244-03, 98132-244-21, 98132-245-03, 98132-245-21, 98132-246-03, 98132-246-21, 98132-247-03, 98132-247-21, 98132-248-03, 98132-248-21, 98132-249-03, 98132-249-21, 98132-250-03, 98132-250-21
- Packager: Orveon Global US LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
- Warning
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bother some dryness or peeling occurs, reduce application to once a day or every other day."
-
Inactive Ingredients
lauroyl lysine, hdi/trimethylol hexyllactone crosspolymer, mica, glycerin, silica, magnesium silicate, kaolin, water, oryza sativa (rice) bran oil, honey extract, boswellia serrata extract, vegetable amino acids, camellia sinensis leaf extract, aspalathus linearis extract, glycyrrhiza glabra (licorice) root extract, butylene glycol, avena sativa (oat) kernel extract, chamomilla recutita (matricaria) flower extract, arnica montana flower extract, sodium hyaluronate, sulfur, stearyl triethoxysilane, maltodextrin, potassium palmitoyl hydrolyzed oat protein, hydrolyzed oat protein, olea europaea (olive) fruit oil, helianthus annuus (sunflower) seed oil, phenoxyethanol, phytosphingosine, panthenol, aloe barbadensis leaf, allantoin, tocopheryl acetate, tetrapeptide-14, beta-glucan, benzoic acid, betaine, lecithin, humic acids, sodium benzoate, dehydroacetic acid, ethylhexylglycerin, chlorphenesin. may contain: titanium dioxide, iron oxides, zinc oxide.
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Fair 1C
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Fair Ivory 1N
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Fairly Light 1 NW
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Fairly Medium 1.5C
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Neutral Ivory 2N
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Light 2W
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Medium 3C
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Soft Medium 2CN
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Medium Beige 2.5N
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Golden Beige 2.5NW
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Neutral Medium 3N
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Golden Nude 3.5NW
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Tan Nude 3.5W
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Medium Tan 3.5CN
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Neutral Tan 4N
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Warm Tan 4.5CN
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Medium Dark 5CN
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Warm Deep 5.5N
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Neutral Deep 5.5NW
- PRINCIPAL DISPLAY PANEL - 6 g Jar Carton - Deepest Deep 6C
-
INGREDIENTS AND APPEARANCE
BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION FAIR 1C
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-231 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) MICA (UNII: V8A1AW0880) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LAUROYL LYSINE (UNII: 113171Q70B) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-231-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-231-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION FAIR IVORY 1N
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-232 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-232-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-232-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION FAIRLY LIGHT 1 NW
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-233 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-233-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-233-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION FAIRLY MEDIUM 1.5C
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-234 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-234-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-234-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION NEUTRAL IVORY 2N
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-235 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-235-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-235-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION LIGHT 2W
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-236 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-236-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-236-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION MEDIUM 3C
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-237 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-237-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-237-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION SOFT MEDIUM 2CN
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-238 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-238-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-238-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION MEDIUM BEIGE 2.5N
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-239 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-239-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-239-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION GOLDEN BEIGE 2.5NW
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-240 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-240-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-240-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION NEUTRAL MEDIUM 3N
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-241 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-241-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-241-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION GOLDEN NUDE 3.5NW
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-242 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-242-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-242-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION TAN NUDE 3.5W
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-243 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-243-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-243-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION MEDIUM TAN 3.5CN
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-244 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-244-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-244-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION NEUTRAL TAN 4N
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-245 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-245-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-245-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION WARM TAN 4.5CN
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-246 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-246-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-246-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION MEDIUM DARK 5CN
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-247 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-247-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-247-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION WARM DEEP 5.5N
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-248 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-248-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-248-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION NEUTRAL DEEP 5.5NW
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-249 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) LAUROYL LYSINE (UNII: 113171Q70B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-249-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-249-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 BAREMINERALS BLEMISH RESCUE SKIN-CLEARING LOOSE FOUNDATION DEEPEST DEEP 6C
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-250 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 6 g Inactive Ingredients Ingredient Name Strength LAUROYL LYSINE (UNII: 113171Q70B) MICA (UNII: V8A1AW0880) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM SILICATE (UNII: 9B9691B2N9) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) RICE BRAN OIL (UNII: LZO6K1506A) HONEY (UNII: Y9H1V576FH) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) CHAMOMILE (UNII: FGL3685T2X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SULFUR (UNII: 70FD1KFU70) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) MALTODEXTRIN (UNII: 7CVR7L4A2D) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) BENZOIC ACID (UNII: 8SKN0B0MIM) BETAINE (UNII: 3SCV180C9W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM BENZOATE (UNII: OJ245FE5EU) DEHYDROACETIC ACID (UNII: 2KAG279R6R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-250-21 1 in 1 CARTON 12/01/2019 1 6 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:98132-250-03 1 in 1 CARTON 12/01/2019 2 0.75 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2019 Labeler - Orveon Global US LLC (118344494)