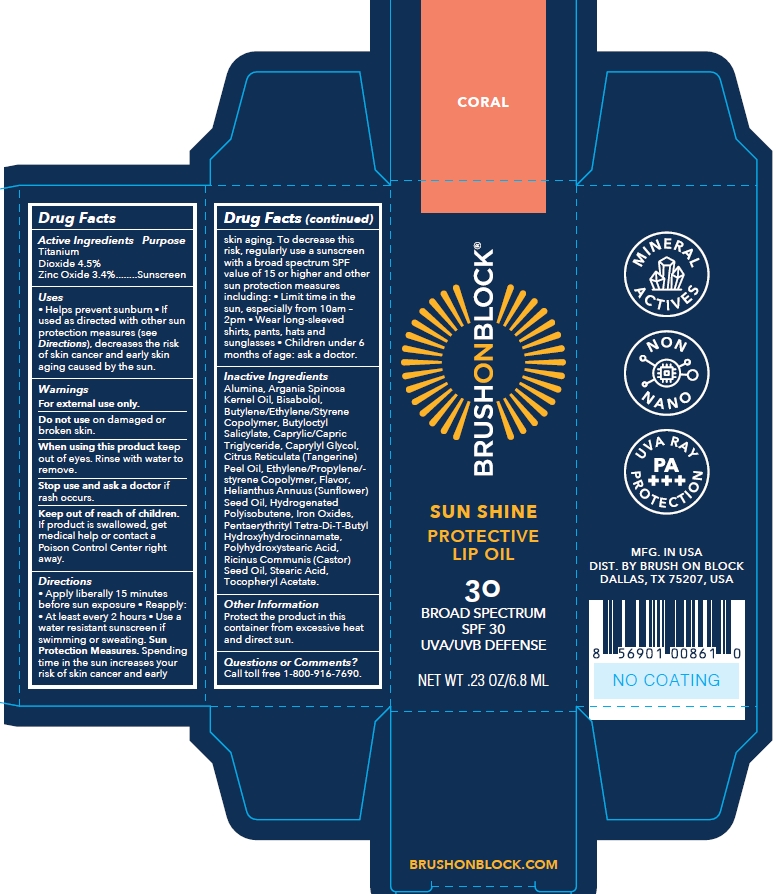
Label: BRUSH ON BLOCK SUN SHINE SPF 30 PROTECTIVE LIP OIL CORAL- titanium dioxide/zinc oxide oil
- NDC Code(s): 58274-017-01
- Packager: SPF Ventures LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure.
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Children under 6 months of age: Ask a doctor.
- Sunprotection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10am-2pm
- wear long-sleeved shirts, pants, hats and sunglasses.
-
Inactive ingredients
ALUMINA
ARGANIA SPINOSA KERNEL OIL
BISABOLOL
BUTYLENE/ETHYLENE/STYRENE COPOLYMER
BUTYLOCTYL SALICYLATE
CAPRYLIC/CAPRIC TRIGLYCERIDE
CAPRYLYL GLYCOL
CITRUS RETICULATA (TANGERINE) PEEL OIL
ETHYLENE/PROPYLENE/STYRENE COPOLYMER
FLAVOR
HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL
HYDROGENATED POLYISOBUTENE
IRON OXIDES
PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE
POLYHYDROXYSTEARIC ACID
RICINUS COMMUNIS (CASTOR) SEED OIL
STEARIC ACID
TOCOPHERYL ACETATE
- Other information
- label
-
INGREDIENTS AND APPEARANCE
BRUSH ON BLOCK SUN SHINE SPF 30 PROTECTIVE LIP OIL CORAL
titanium dioxide/zinc oxide oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58274-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 4.5 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 3.4 g in 100 mL Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ARGAN OIL (UNII: 4V59G5UW9X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM OXIDE (UNII: LMI26O6933) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) STEARIC ACID (UNII: 4ELV7Z65AP) MANDARIN OIL (UNII: NJO720F72R) LEVOMENOL (UNII: 24WE03BX2T) SUNFLOWER OIL (UNII: 3W1JG795YI) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYL PALMITATE (UNII: 2865993309) Product Characteristics Color Score Shape Size Flavor TANGERINE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58274-017-01 6.8 mL in 1 TUBE; Type 0: Not a Combination Product 03/05/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/05/2024 Labeler - SPF Ventures LLC (055483891) Establishment Name Address ID/FEI Business Operations Dermaceutical Laboratories Limited Liability 078457159 manufacture(58274-017) Establishment Name Address ID/FEI Business Operations Dermaceutical Laboratories Limited Liability 080375664 manufacture(58274-017)