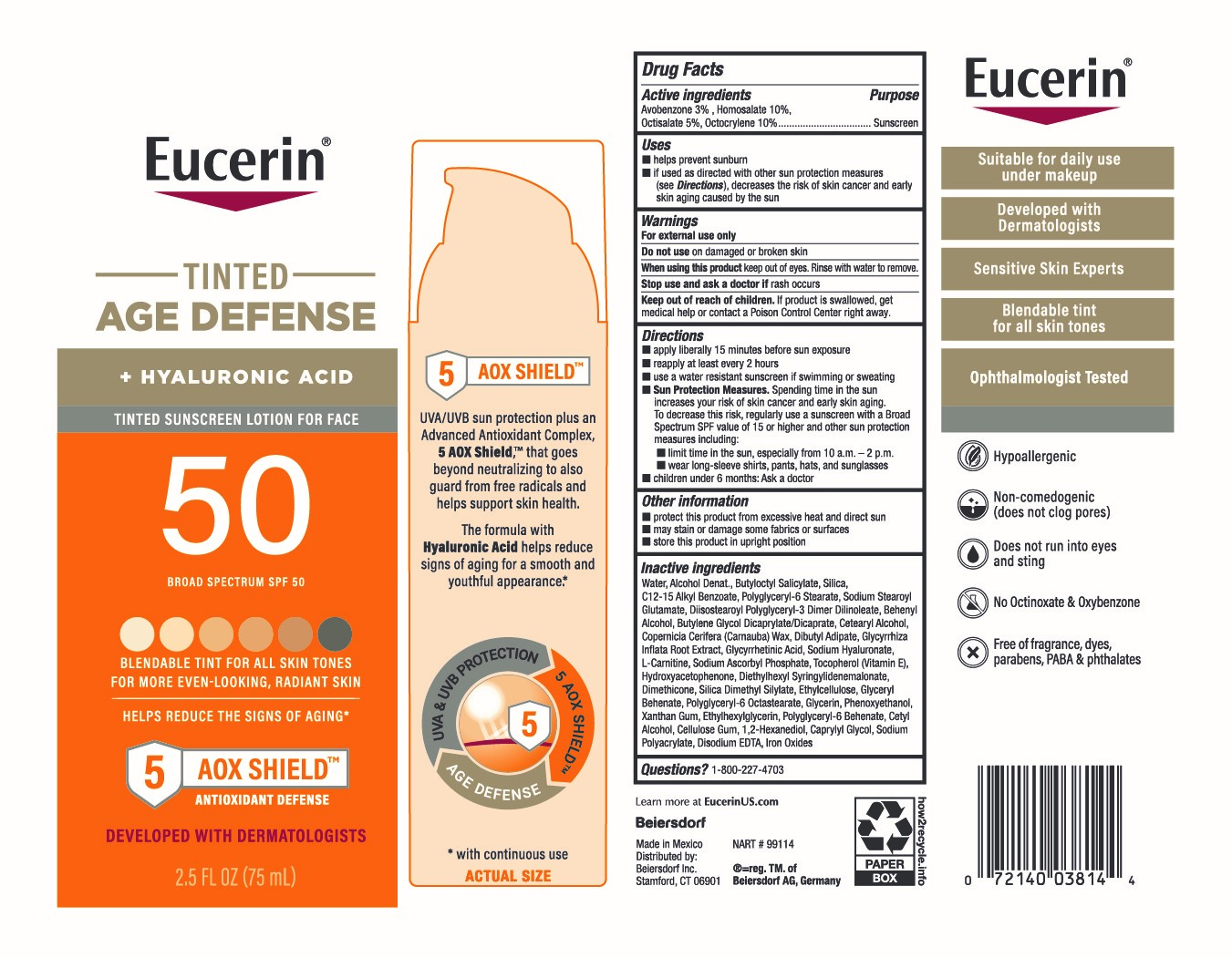
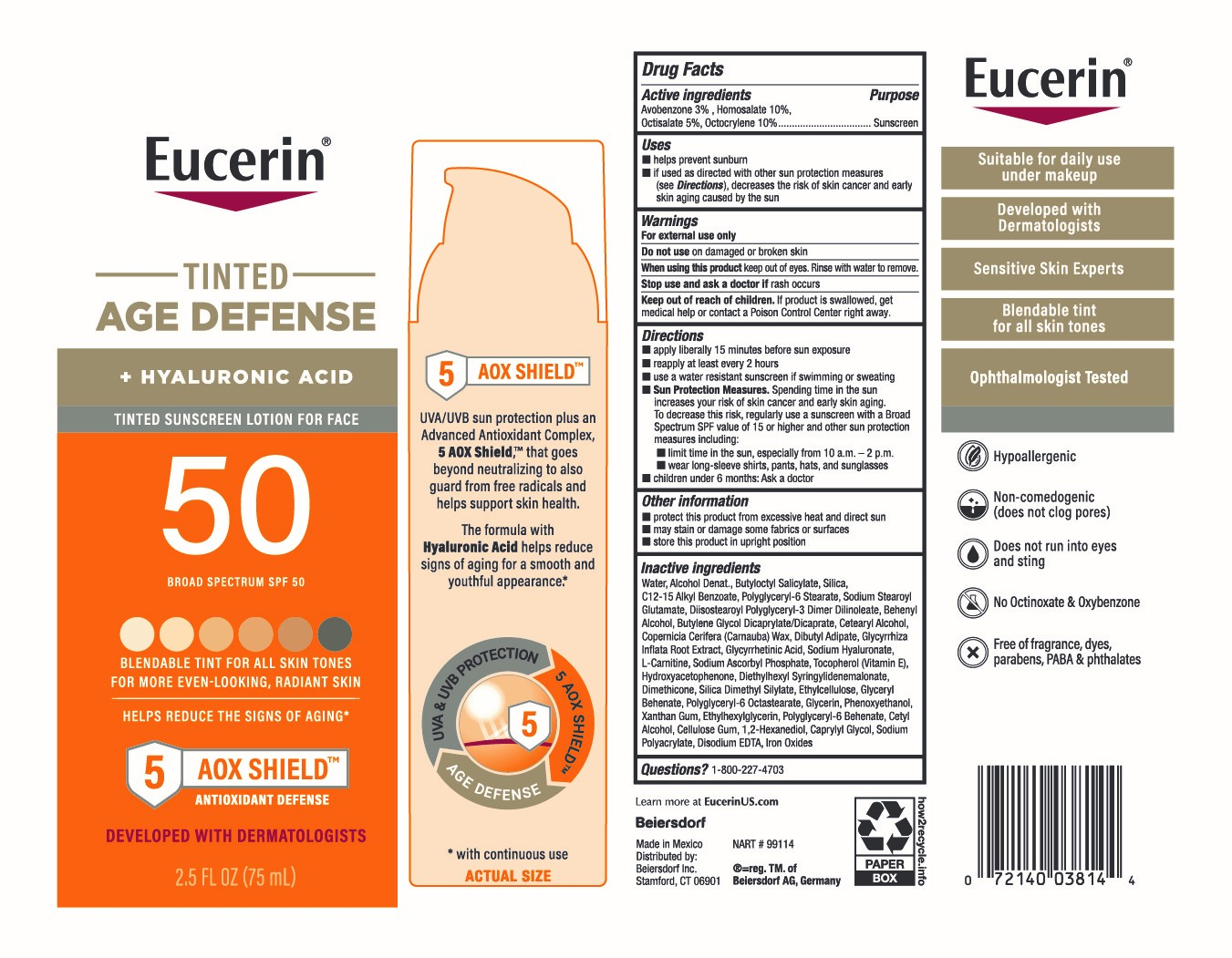
Label: EUCERIN TINTED AGE DEFENSE SUNCREEN SPF 50- avobenzone 3%, homosalate 10%, octisalate 5%, octocrylene 10% lotion
- NDC Code(s): 66800-0381-3, 66800-0381-4
- Packager: Beiersdorf Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
■ apply liberally 15 minutes before sun exposure
■ reapply at least every 2 hours
■ use a water resistant sunscreen if swimming or sweating
■ Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
■ limit time in the sun, especially from 10 a.m. – 2 p.m.
■ wear long-sleeve shirts, pants, hats, and sunglasses
■ children under 6 months: Ask a doctor
- Other information
-
Inactive ingredients
Water, Alcohol Denat., Butyloctyl Salicylate, Silica, C12-15 Alkyl Benzoate, Polyglyceryl-6 Stearate, Sodium Stearoyl Glutamate, Diisostearoyl Polyglyceryl-3 Dimer Dilinoleate, Behenyl Alcohol, Butylene Glycol Dicaprylate/Dicaprate, Cetearyl Alcohol, Copernicia Cerifera (Carnauba) Wax, Dibutyl Adipate, Glycyrrhiza Inflata Root Extract, Glycyrrhetinic Acid, Sodium Hyaluronate, L-Carnitine, Sodium Ascorbyl Phosphate, Tocopherol (Vitamin E), Hydroxyacetophenone, Diethylhexyl Syringylidenemalonate, Dimethicone, Silica Dimethyl Silylate, Ethylcellulose, Glyceryl Behenate, Polyglyceryl-6 Octastearate, Glycerin, Phenoxyethanol, Xanthan Gum, Ethylhexylglycerin, Polyglyceryl-6 Behenate, Cetyl Alcohol, Cellulose Gum, 1,2-Hexanediol, Caprylyl Glycol, Sodium Polyacrylate, Disodium EDTA, Iron Oxides
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EUCERIN TINTED AGE DEFENSE SUNCREEN SPF 50
avobenzone 3%, homosalate 10%, octisalate 5%, octocrylene 10% lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66800-0381 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 g Inactive Ingredients Ingredient Name Strength DL-CARNITINE (UNII: S7UI8SM58A) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) ALLANTOIN GLYCYRRHETINIC ACID (UNII: H6FL381368) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) BROWN IRON OXIDE (UNII: 1N032N7MFO) GLYCERIN (UNII: PDC6A3C0OX) DOCOSANOL (UNII: 9G1OE216XY) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) POLYGLYCERYL-6 BEHENATE (UNII: 4T2L7QI313) DISODIUM EDTA-COPPER (UNII: 6V475AX06U) WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DIBUTYL ADIPATE (UNII: F4K100DXP3) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POLYGLYCERYL-6 STEARATE (UNII: ETY9Q81E2T) DIISOSTEAROYL POLYGLYCERYL-3 DIMER DILINOLEATE (UNII: G3232Z5S2O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCYRRHIZA INFLATA ROOT (UNII: 1MV1Z7MKVQ) XANTHAN GUM (UNII: TTV12P4NEE) CETYL ALCOHOL (UNII: 936JST6JCN) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALCOHOL (UNII: 3K9958V90M) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) DIMETHICONE (UNII: 92RU3N3Y1O) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYLGLYCERYL BEHENATE (UNII: JRY34LRD7B) Product Characteristics Color white (White to Off-White) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66800-0381-4 1 in 1 CARTON 03/01/2024 1 75 g in 1 CAN; Type 0: Not a Combination Product 2 NDC:66800-0381-3 10 g in 1 PACKET; Type 0: Not a Combination Product 03/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/30/2023 Labeler - Beiersdorf Inc (001177906)