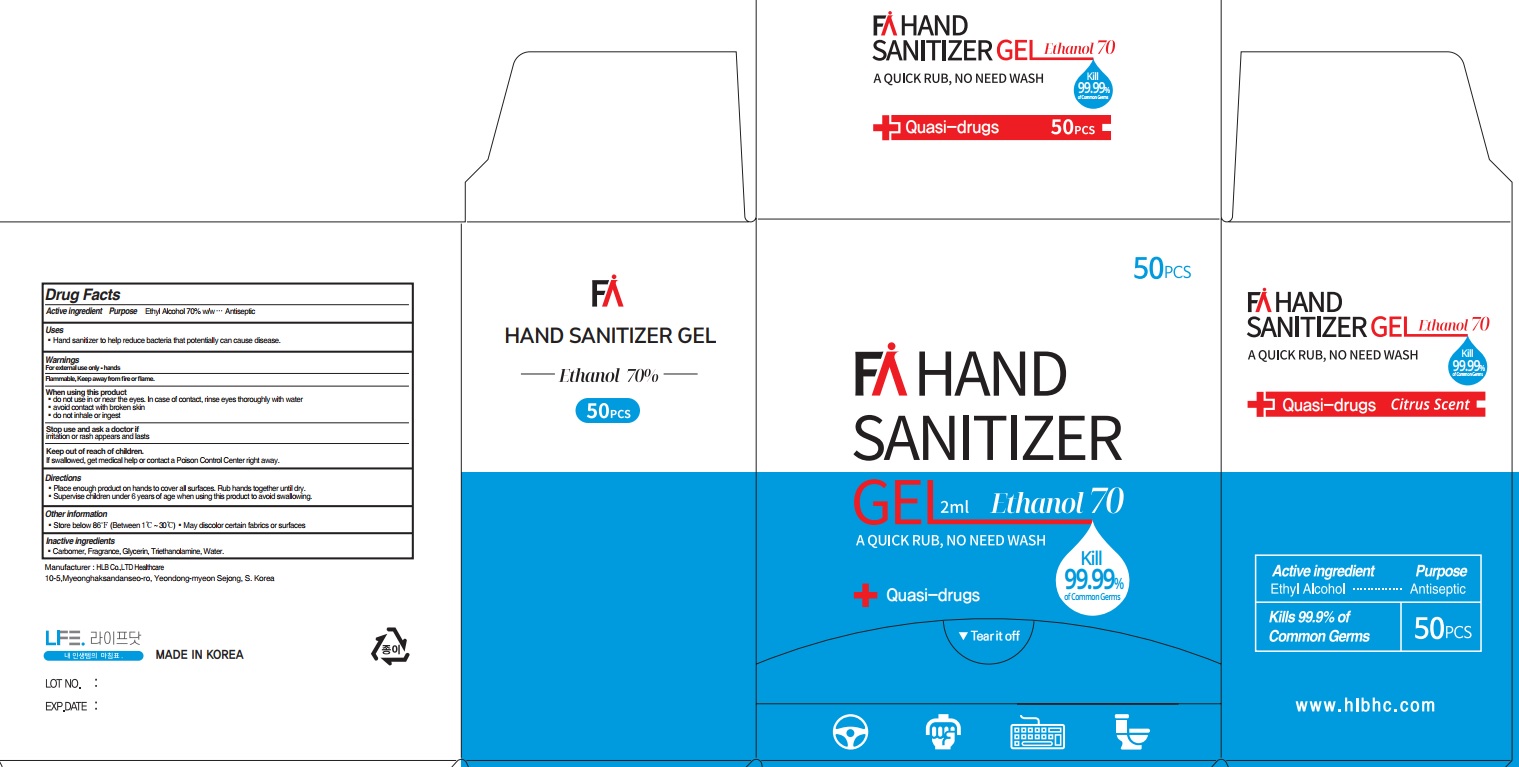
Label: FA HAND SANITIZER- ethyl alcohol gel
- NDC Code(s): 74932-320-01, 74932-320-02, 74932-320-03, 74932-320-04
- Packager: HLB CO.,LTD_Healthcare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENTS
- PURPOSE
-
WARNINGS
For external use only. Flammable. Keep away from heat or flame
--------------------------------------------------------------------------------------------------------
When using this product ■ do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water ■ avoid contact with broken skin ■ do not inhale or ingest
--------------------------------------------------------------------------------------------------------
Stop use and ask a doctor if irritation or rash appears and lasts - KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- Other Information
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL (2mL*50pcs)
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL (500mL Bottle)
-
INGREDIENTS AND APPEARANCE
FA HAND SANITIZER
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74932-320 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Alcohol (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) Alcohol 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) Glycerin (UNII: PDC6A3C0OX) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74932-320-02 50 in 1 CARTON 06/01/2020 1 NDC:74932-320-01 2 mL in 1 POUCH; Type 0: Not a Combination Product 2 NDC:74932-320-03 300 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2020 12/30/2022 3 NDC:74932-320-04 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/01/2020 Labeler - HLB CO.,LTD_Healthcare (987587913) Registrant - HLB CO.,LTD_Healthcare (987587913) Establishment Name Address ID/FEI Business Operations HLB CO.,LTD_Healthcare 987587913 manufacture(74932-320)