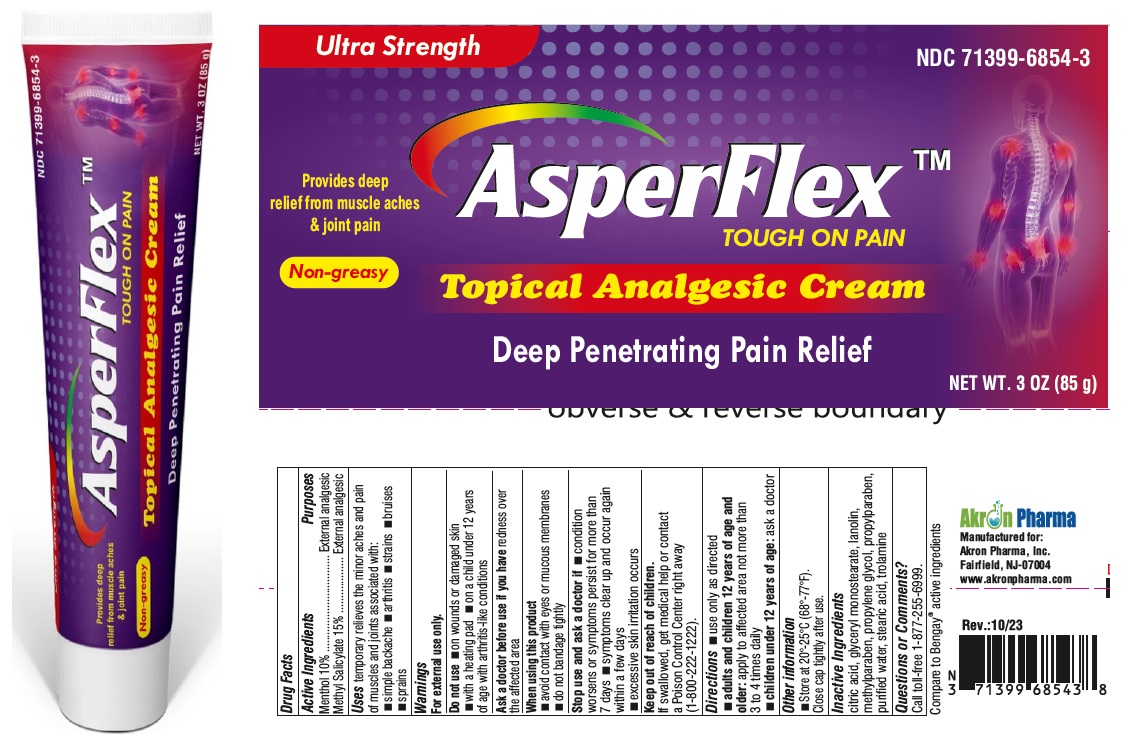
Label: ASPERFLEX- menthol and methyl salicylate cream
- NDC Code(s): 71399-6854-3
- Packager: Akron Pharma Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- on wounds or damaged skin
- with a heating pad
- on a child under 12 years of age with arthritis-like conditions
- Directions
- Other Information
- Inactive ingredients
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ASPERFLEX
menthol and methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71399-6854 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 100 mg in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 150 mg in 1 g Inactive Ingredients Ingredient Name Strength DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) CITRIC ACID ACETATE (UNII: DSO12WL7AU) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LANOLIN (UNII: 7EV65EAW6H) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71399-6854-3 85 g in 1 TUBE; Type 0: Not a Combination Product 06/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/03/2024 Labeler - Akron Pharma Inc. (067878881)