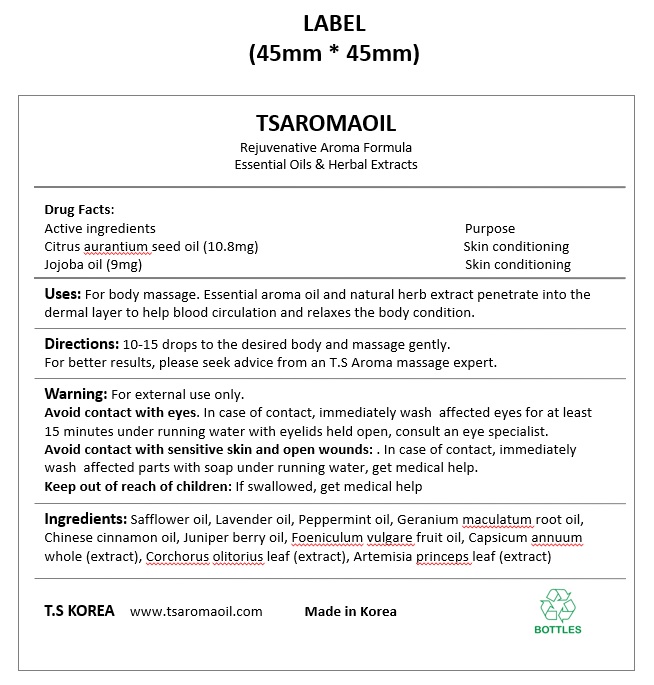
Label: TSAROMAOIL- citrus aurantium seed oil, jojoba oil oil
- NDC Code(s): 83911-201-01
- Packager: TS KOREA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
WARNINGS
Warning: For external use only.
Avoid contact with eyes. In case of contact, immediately wash affected eyes for at least 15 minutes under running water with eyelids held open, consult an eye specialist.
Avoid contact with sensitive skin and open wounds: . In case of contact, immediately wash affected parts with soap under running water, get medical help.
Keep out of reach of children: If swallowed, get medical help
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TSAROMAOIL
citrus aurantium seed oil, jojoba oil oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83911-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength JOJOBA OIL (UNII: 724GKU717M) (JOJOBA OIL - UNII:724GKU717M) JOJOBA OIL 9 mg in 100 mL CITRUS AURANTIFOLIA SEED OIL (UNII: EW089HAI77) (CITRUS AURANTIFOLIA SEED OIL - UNII:EW089HAI77) CITRUS AURANTIFOLIA SEED OIL 10.8 mg in 100 mL Inactive Ingredients Ingredient Name Strength GERANIUM MACULATUM ROOT OIL (UNII: H2E371EDYX) FOENICULUM VULGARE FRUIT OIL (UNII: 91UAY89ODU) CORCHORUS OLITORIUS LEAF (UNII: 1FG47O9LG5) SAFFLOWER OIL (UNII: 65UEH262IS) LAVENDER OIL (UNII: ZBP1YXW0H8) JUNIPER BERRY OIL (UNII: SZH16H44UY) ARTEMISIA PRINCEPS LEAF (UNII: SY077EW02G) CAPSICUM ANNUUM WHOLE (UNII: 7FKZ3QQQ1F) PEPPERMINT OIL (UNII: AV092KU4JH) CHINESE CINNAMON OIL (UNII: A4WO0626T5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83911-201-01 50 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 11/22/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/22/2023 Labeler - TS KOREA (690114435) Registrant - TS KOREA (690114435) Establishment Name Address ID/FEI Business Operations TS KOREA 690114435 manufacture(83911-201)