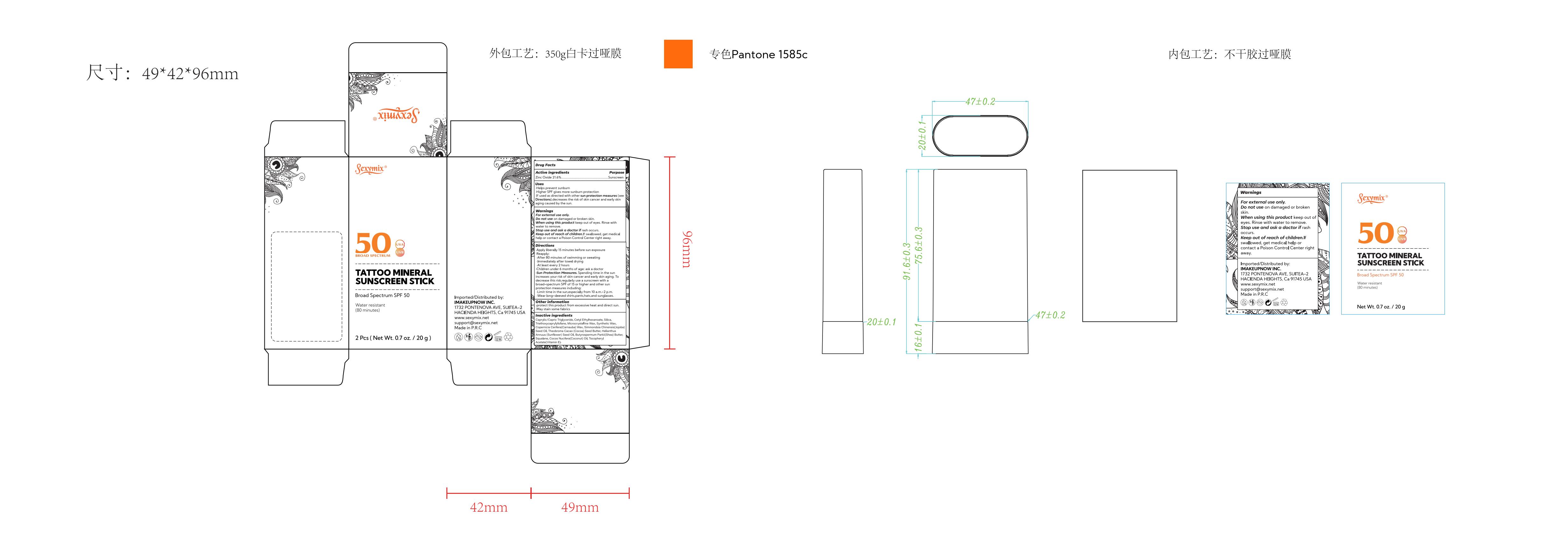
Label: SEXY MIX TATTOO MINERAL SUNSCREEN STICK BROAD SPECTRUM SPF 50- zinc oxide stick
- NDC Code(s): 82723-004-01
- Packager: Aopline Health Industry Technology (Guangzhou) Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
Directions
Apply liberally 15 minutes before sun exposure
Reappl.
After 80 minutes of swimming or sweating
Immediately after towel drying
At least every 2 hours-
Children under 6 months of age . ask a doctor-
Sun Protection Measures . Spending time in the sun
increases your risk of skin cancer and early skin agng . To
decrease this risk , regularly use a sunscreen with a
broad-spectrum SPF of 15 or higher and other sun
protection measures including
Limit time in the sun , especially from 10 a.m. -2 p m.
Wear long-sleeved shirts , pants , hats , and sunglasses - Other Information
-
Inactive Ingredients
Inactive ingredient
Caprylic / Capric Triglyceride , Cetyl Ethylhexanoate , Silica ,
TriethoxycaprylIylsilane , Microcrystalline Wax , Synthetic Wax ,
Copernicia Cerifera ( Carnauba ) Wax , Simmondsia Chinensis ( Jojoba)
Seed Oil , Theobroma Cacao ( Cocoa ) Seed Butter , Helianthus
Annuus ( Sunflower ) Seed Oil , Butyrospermum Parki Shea ) Butter ,
Squalane , Cocos Nucifera ( Coconut ) Oil , Tocopheryl
Acetate ( Vitamin E) - Keep out of reach of children.
- Stop use
- WHEN USING
- Do not use
- Package Label
-
INGREDIENTS AND APPEARANCE
SEXY MIX TATTOO MINERAL SUNSCREEN STICK BROAD SPECTRUM SPF 50
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82723-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 21.6 g in 100 g Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SUNFLOWER OIL (UNII: 3W1JG795YI) SHEA BUTTER (UNII: K49155WL9Y) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SQUALANE (UNII: GW89575KF9) COCONUT OIL (UNII: Q9L0O73W7L) JOJOBA OIL (UNII: 724GKU717M) COCOA BUTTER (UNII: 512OYT1CRR) CARNAUBA WAX (UNII: R12CBM0EIZ) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82723-004-01 20 g in 1 BOX; Type 0: Not a Combination Product 11/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2023 Labeler - Aopline Health Industry Technology (Guangzhou) Co., Ltd. (715076108) Establishment Name Address ID/FEI Business Operations Aopline Health Industry Technology (Guangzhou) Co., Ltd. 715076108 manufacture(82723-004)