Label: PHYTOGENIC INFINTE 37100457- octinoxate, titanium dioxide and zinc oxide cream cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 51523-457-35 - Packager: THEFACESHOP CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 20, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
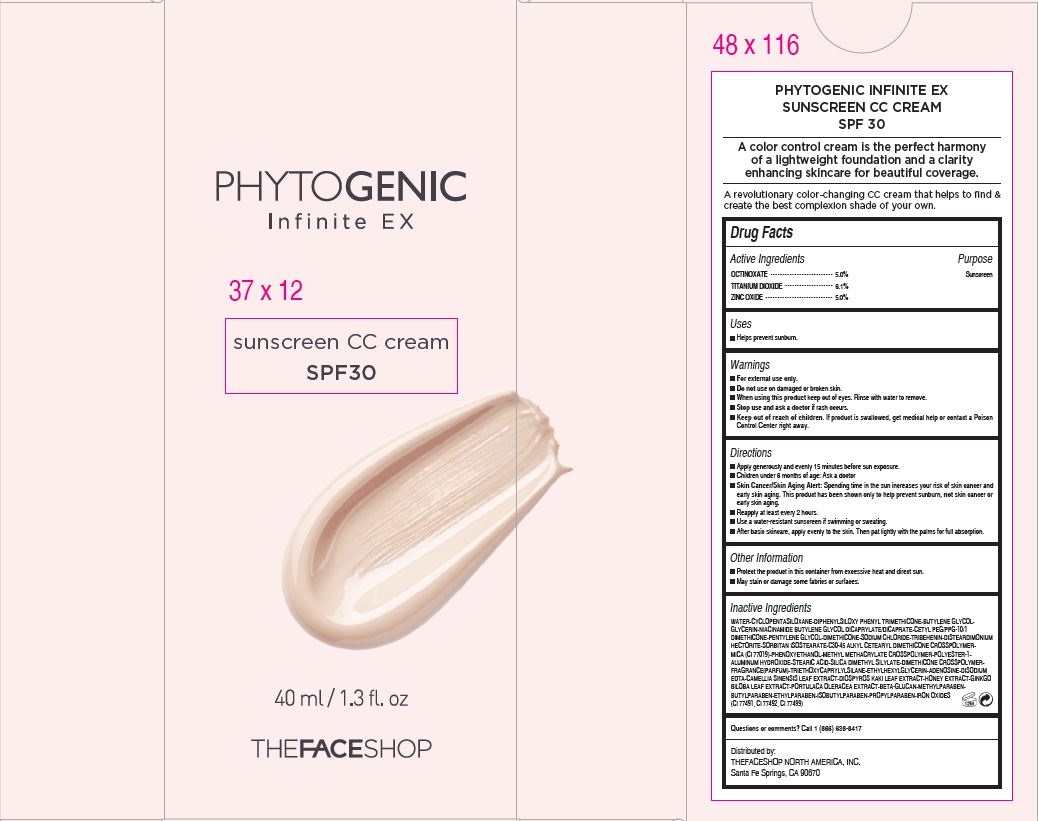
- Active Ingredients
- Purpose
- Warnings
-
Directions
Apply generously and evenly 15 minutes before sun exposure.
Children under 6 months of age: Ask a doctor
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and
early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or
early skin aging.
Reapply at least every 2 hours.
Use a water-resistant sunscreen if swimming or sweating.
After basic skincare, apply evenly to the skin. Then pat lightly with the palms for full absorption. - Other Information
-
Inactive Ingredients
WATER•CYCLOPENTASILOXANE•DIPHENYLSILOXY PHENYL TRIMETHICONE•BUTYLENE GLYCOL•
GLYCERIN•NIACINAMIDE BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE•CETYL PEG/PPG-10/1
DIMETHICONE•PENTYLENE GLYCOL•DIMETHICONE•SODIUM CHLORIDE•TRIBEHENIN•DISTEARDIMONIUM
HECTORITE•SORBITAN ISOSTEARATE•C30-45 ALKYL CETEARYL DIMETHICONE CROSSPOLYMER•
MICA (CI 77019)•PHENOXYETHANOL•METHYL METHACRYLATE CROSSPOLYMER•POLYESTER-1•
ALUMINUM HYDROXIDE•STEARIC ACID•SILICA DIMETHYL SILYLATE•DIMETHICONE CROSSPOLYMER•
FRAGRANCE(PARFUM)•TRIETHOXYCAPRYLYLSILANE•ETHYLHEXYLGLYCERIN•ADENOSINE•DISODIUM
EDTA•CAMELLIA SINENSIS LEAF EXTRACT•DIOSPYROS KAKI LEAF EXTRACT•HONEY EXTRACT•GINKGO
BILOBA LEAF EXTRACT•PORTULACA OLERACEA EXTRACT•BETA-GLUCAN•METHYLPARABEN•
BUTYLPARABEN•ETHYLPARABEN•ISOBUTYLPARABEN•PROPYLPARABEN•IRON OXIDES
(CI 77491, CI 77492, CI 77499) - QUESTIONS
- Distributed by:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PHYTOGENIC INFINTE 37100457
octinoxate, titanium dioxide and zinc oxide cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51523-457 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2 g in 40 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2 g in 40 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.4 g in 40 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51523-457-35 1 in 1 CARTON 11/20/2014 1 40 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/20/2014 Labeler - THEFACESHOP CO., LTD. (688329416) Registrant - THEFACESHOP NORTH AMERICA, INC. (620459193) Establishment Name Address ID/FEI Business Operations THEFACESHOP Co., Ltd. 688329416 label(51523-457)