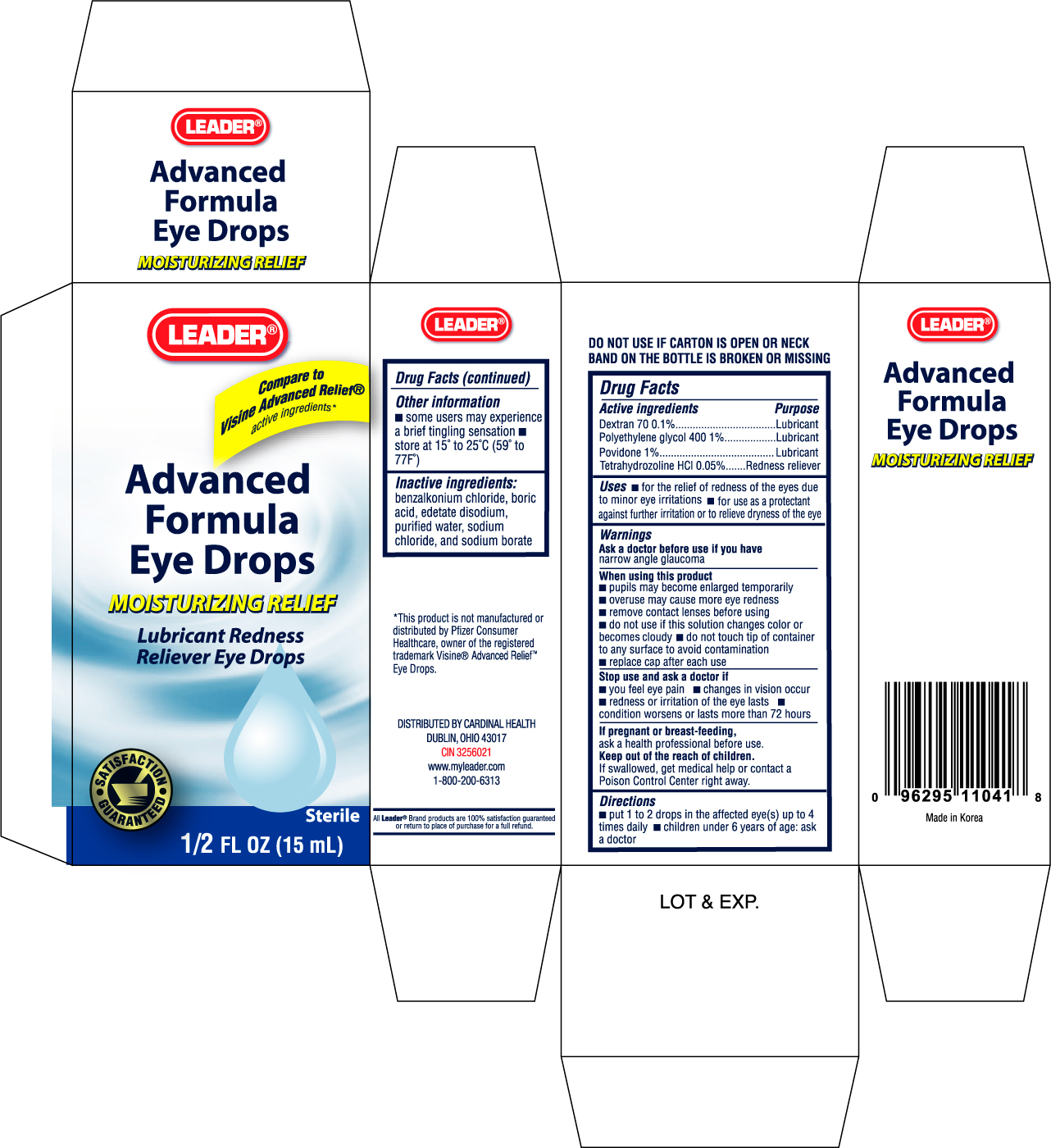
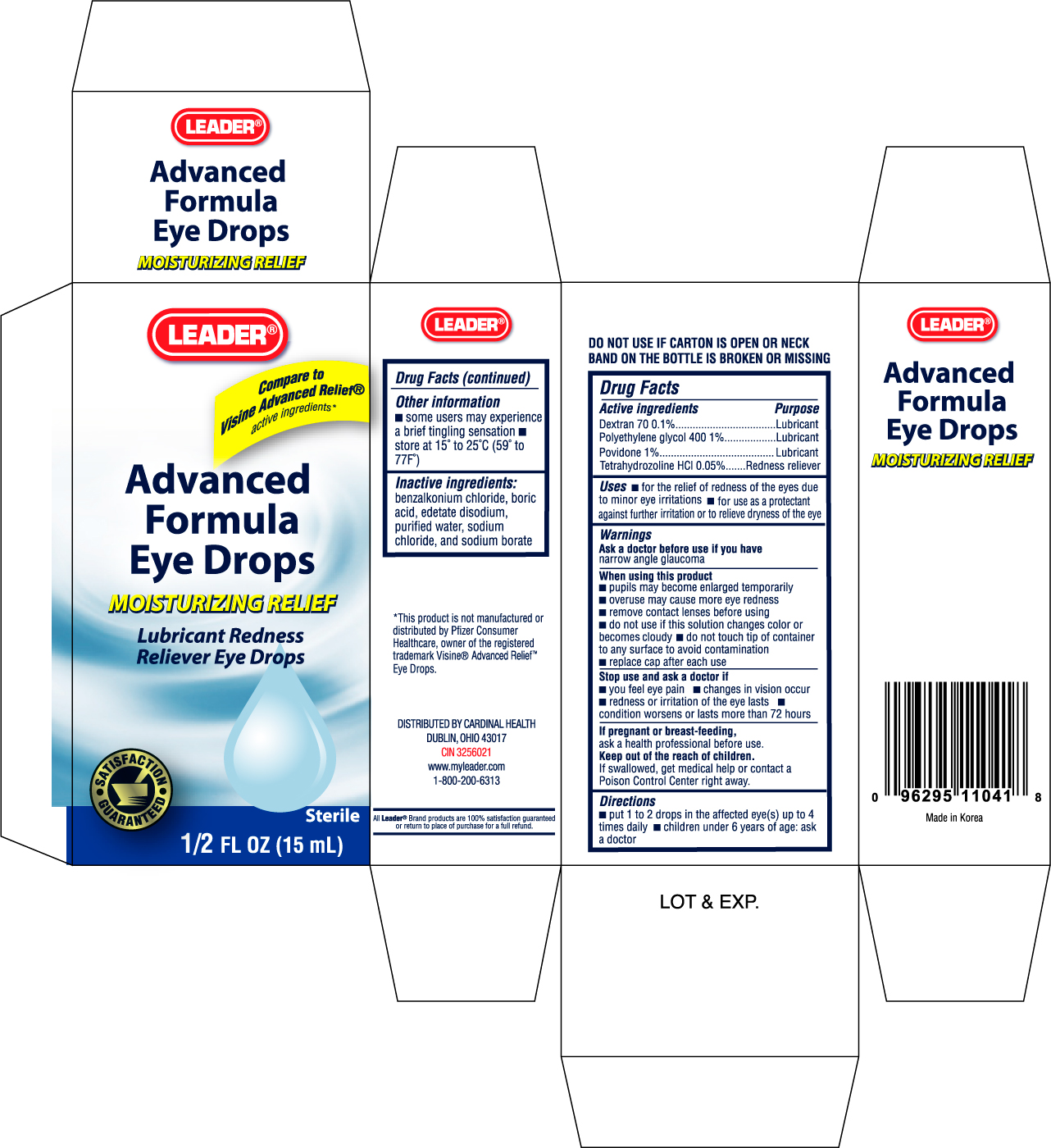
Label: LEADER ADVANCED FORMULA EYE DROPS- dextran 70 solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 11716-1104-1 - Packager: HANLIM PHARM. CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 6, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTActive ingredients Purpose - Dextran 70 0.1%.................................................................Lubricant - Polyethylene ...
-
PURPOSEUses - for the relief of redness of the eyes due to minor eye irritations - for use as a protectant against further irritation or to relieve dryness of the eye
-
WARNINGSWarnings - Ask a doctor before use if you have narrow angle glaucoma
-
WHEN USINGWhen using this product - pupils may become enlarged temporarily - overuse may cause more eye redness - remove contact lenses before using - do not use if this solution changes color or becomes cloudy - do ...
-
STOP USEStop use and ask a doctor if - you feel eye pain - changes in vision occur - redness or irritation of the eye lasts - condition worsens or lasts more than 72 hours
-
PREGNANCY OR BREAST FEEDINGIf pregnant or breast-feeding, ask a health professional before use.
-
KEEP OUT OF REACH OF CHILDRENKeep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
-
INDICATIONS & USAGEDirections - put 1 to 2 drops in the affected eye(s) up to 4 times daily - children under 6 years of age: ask a doctor
-
STORAGE AND HANDLINGOther information - some users may experience a brief tingling sensation - store at 15o to 25oC (59o to 77oF)
-
INACTIVE INGREDIENTInactive ingredients: benzalkonium chloride, boric acid, edetate disodium, purified water, sodium chloride, and sodium borate
-
PRINCIPAL DISPLAY PANELEnter section text here
-
INGREDIENTS AND APPEARANCEProduct Information

 Enter section text here
Enter section text here