Label: MEGABABE BUTT STUFF HEMORRHOIDAL- cocoa butter, glycerin, lidocaine, petrolatum, phenylephrine hydrochloride ointment
- NDC Code(s): 84000-502-00
- Packager: MEGABABE, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
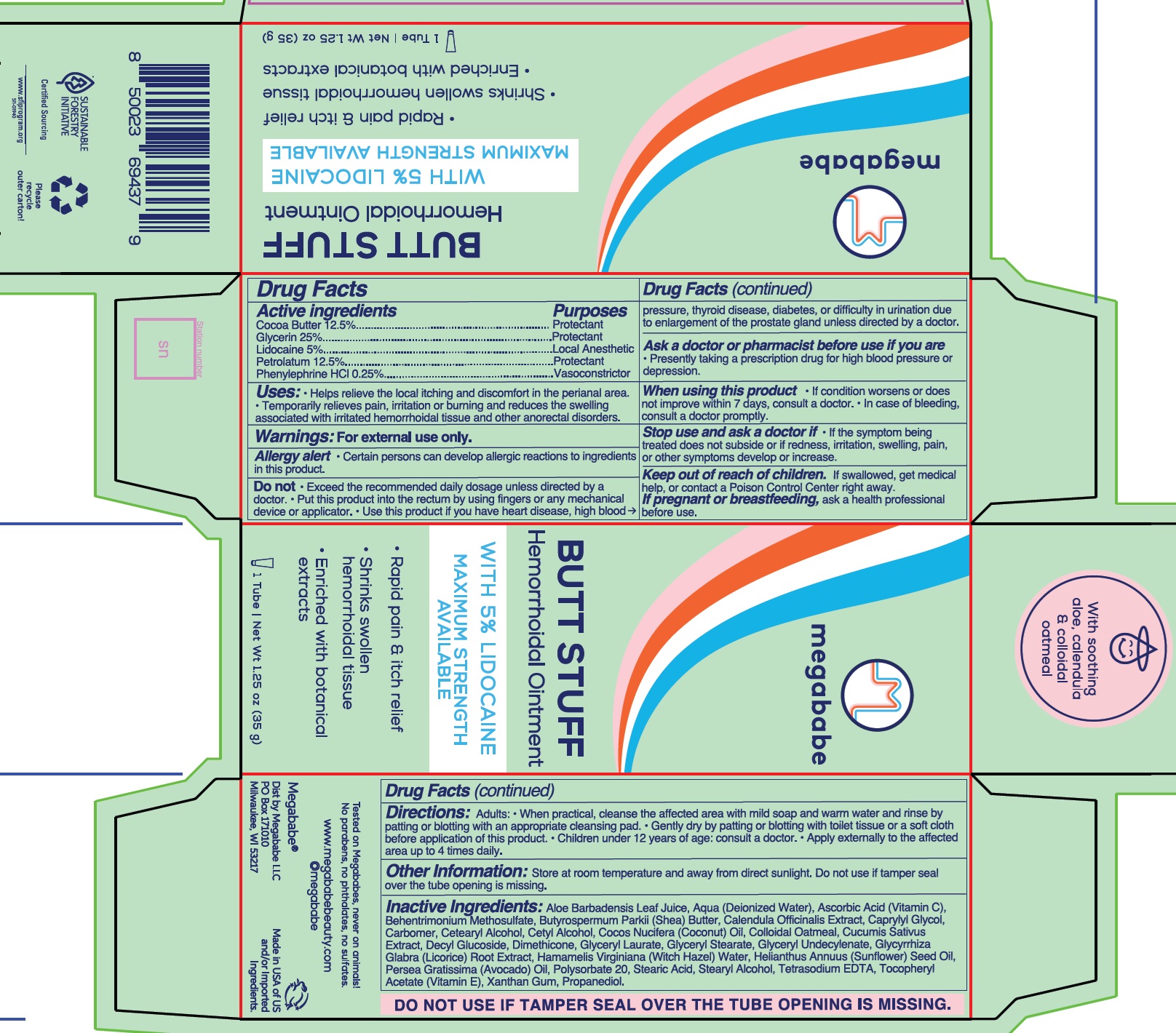
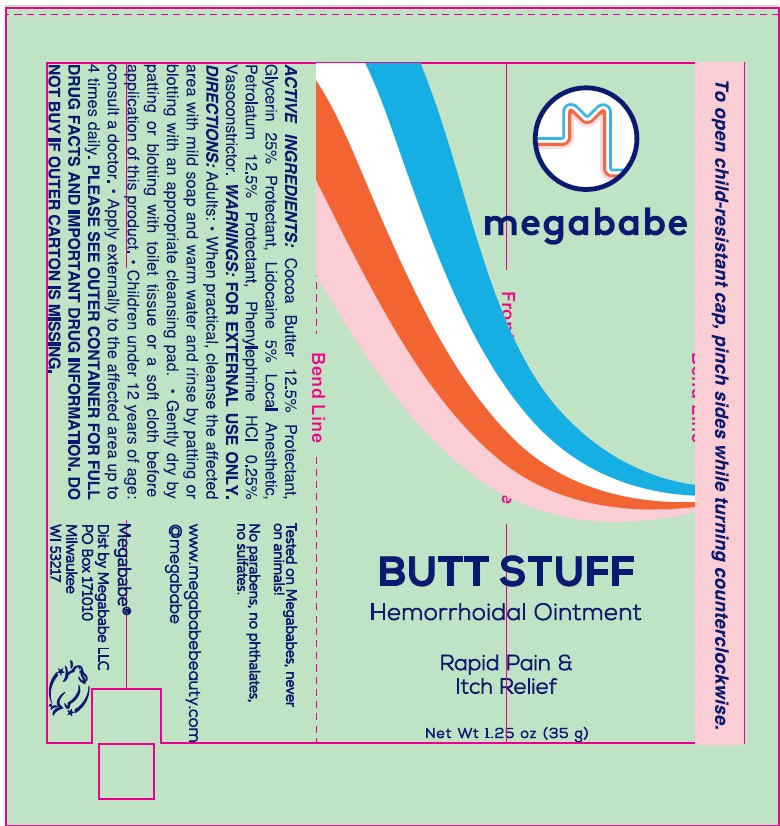
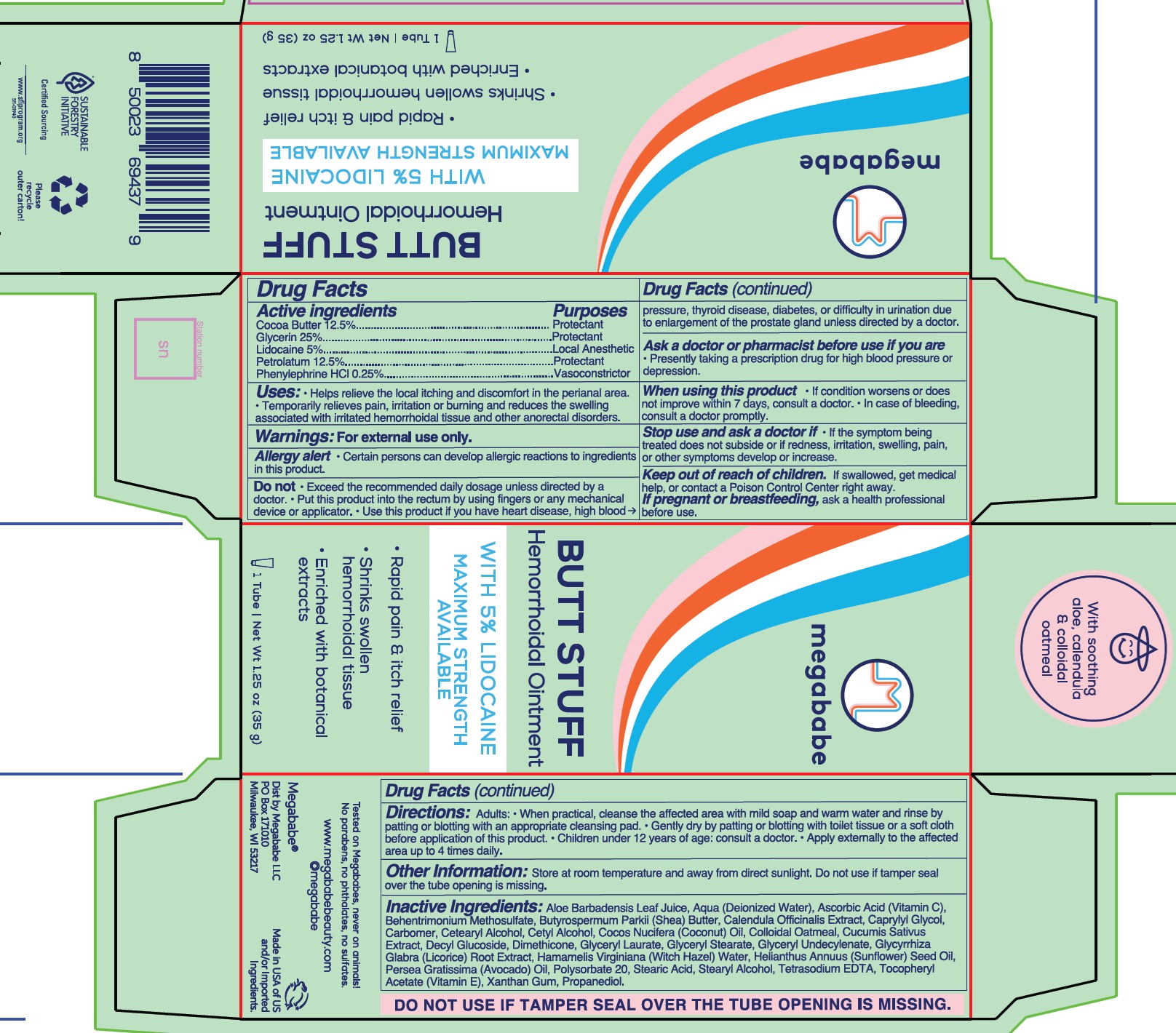
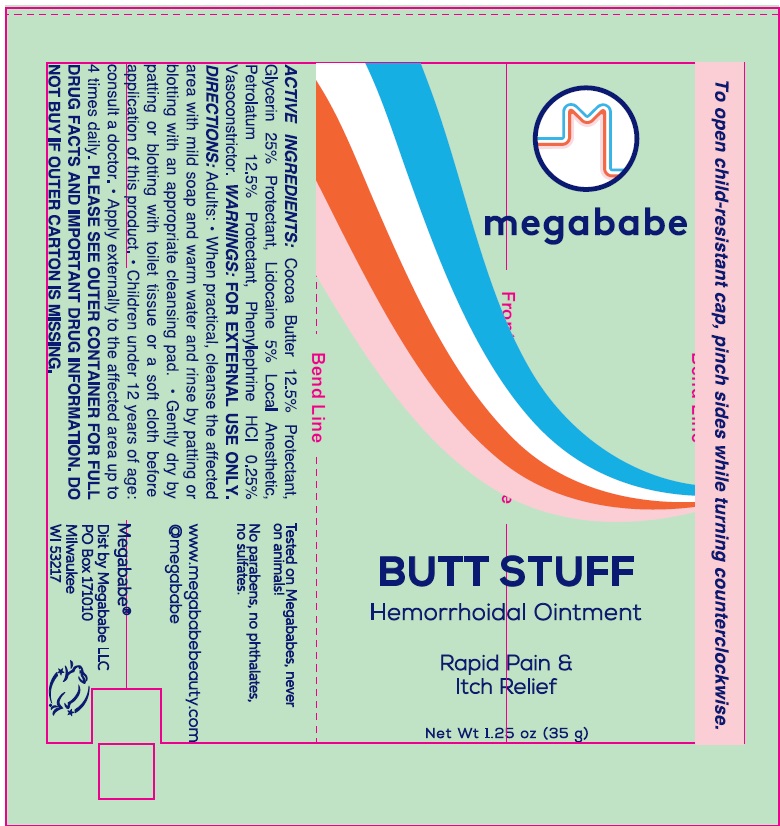
- Drug Facts
- Active ingredients
- Uses:
-
Warnings:
For external use only.
Allergy alert • Certain persons can develop allergic reactions to ingredients in this product.
Do not
- Exceed the recommended daily dosage unless directed by a doctor.
- Put this product into the rectum by using fingers or any mechanical device or applicator.
- Use this product if you have heart disease, high blood pressure, thyroid disease, diabetes, or difficulty in urination due to enlargement of the prostate gland unless directed by a doctor.
Ask a doctor or pharmacist before use if you are
- Presently taking a prescription drug for high blood pressure or depression.
When using this product
- If condition worsens or does not improve within 7 days, consult a doctor.
- In case of bleeding, consult a doctor promptly.
Stop use and ask a doctor if
- If the symptom being treated does not subside or if redness, irritation, swelling, pain, or other symptoms develop or increase.
-
Directions:
Adults: • When practical, cleanse the affected area with mild soap and warm water and rinse by patting or blotting with an appropriate cleansing pad.
- Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product.
- Children under 12 years of age: consult a doctor.
- Apply externally to the affected area up to 4 times daily.
- Other Information:
-
Inactive Ingredients:
Aloe Barbadensis Leaf Juice, Aqua (Deionized Water), Ascorbic Acid (Vitamin C), Behentrimonium Methosulfate, Butyrospermum Parkii (Shea) Butter, Calendula Officinalis Extract, Caprylyl Glycol, Carbomer, Cetearyl Alcohol, Cetyl Alcohol, Cocos Nucifera (Coconut) Oil, Colloidal Oatmeal, Cucumis Sativus Extract, Decyl Glucoside, Dimethicone, Glyceryl Laurate, Glyceryl Stearate, Glyceryl Undecylenate, Glycyrrhiza Glabra (Licorice) Root Extract, Hamamelis Virginiana (Witch Hazel) Water, Helianthus Annuus (Sunflower) Seed Oil, Persea Gratissima (Avocado) Oil, Polysorbate 20, Stearic Acid, Stearyl Alcohol, Tetrasodium EDTA, Tocopheryl Acetate (Vitamin E), Xanthan Gum, Propanediol.
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
MEGABABE BUTT STUFF HEMORRHOIDAL
cocoa butter, glycerin, lidocaine, petrolatum, phenylephrine hydrochloride ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84000-502 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COCOA BUTTER (UNII: 512OYT1CRR) (COCOA BUTTER - UNII:512OYT1CRR) COCOA BUTTER 125 mg in 1 g GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 250 mg in 1 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 50 mg in 1 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 125 mg in 1 g PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) ASCORBIC ACID (UNII: PQ6CK8PD0R) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) SHEA BUTTER (UNII: K49155WL9Y) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) COCONUT OIL (UNII: Q9L0O73W7L) OATMEAL (UNII: 8PI54V663Y) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL LAURATE (UNII: Y98611C087) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) SUNFLOWER OIL (UNII: 3W1JG795YI) AVOCADO OIL (UNII: 6VNO72PFC1) POLYSORBATE 20 (UNII: 7T1F30V5YH) STEARIC ACID (UNII: 4ELV7Z65AP) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) EDETATE SODIUM (UNII: MP1J8420LU) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) XANTHAN GUM (UNII: TTV12P4NEE) PROPANEDIOL (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84000-502-00 1 in 1 BOX 06/06/2024 1 35 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 06/06/2024 Labeler - MEGABABE, LLC (081154311)