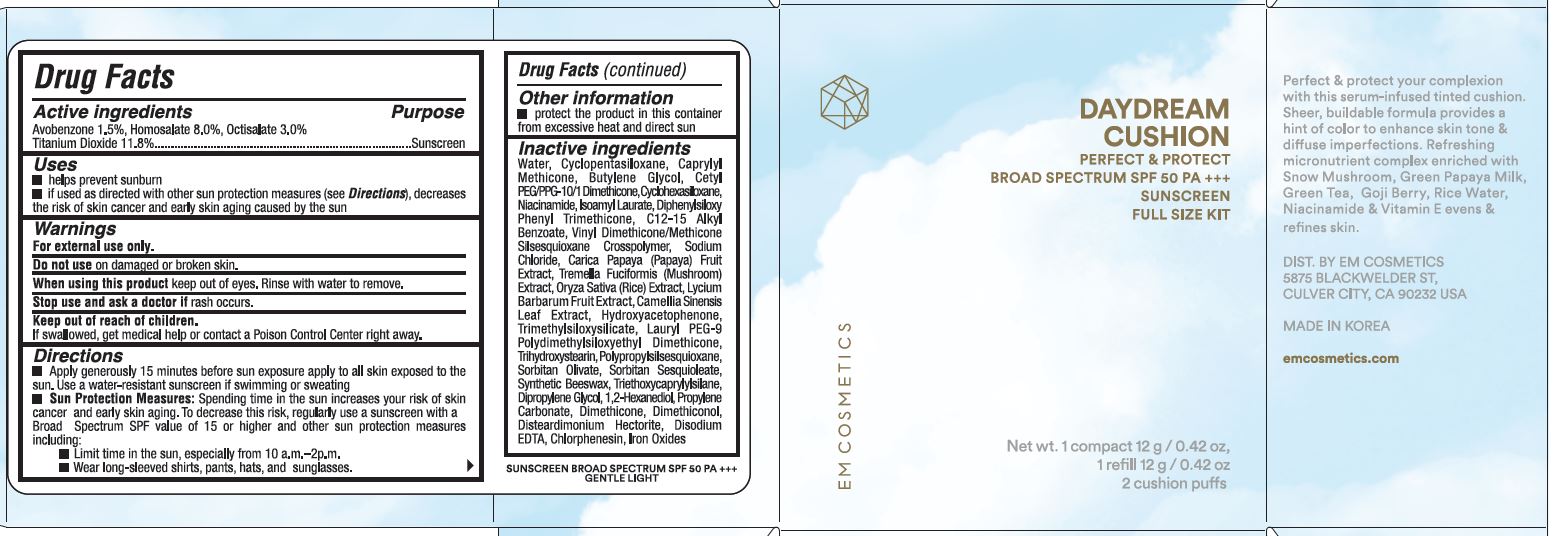
Label: DAYDREAM CUSHION GENTLE LIGHT- avobenzone, homosalate, octisalate, titanium dioxide cream
- NDC Code(s): 73586-103-11
- Packager: EM Cosmetics LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTSAvobenzone 1.5% Homosalate 8.0% Octisalate 3.0% Titanium Dioxide 11.8% Purpose - Sunscreen
-
PurposeSunscreen
-
USESHelps prevent sunburn. If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer, early skin aging by the sun.
-
WARNINGSFor external use only. Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash occurs. Keep out of reach ...
-
DIRECTIONSApply generously 15 minutes before sun exposure apply to all skin exposed to the sun. Use a water-resistant sunscreen if swimming or sweating. Sun Protection Measures: Spending time in the sun ...
-
OTHER INFORMATIONPROTECT THE PRODUCT IN THIS CONTAINER FROM EXCESSIVE HEAT AND DIRECT SUN
-
INACTIVE INGREDIENTSWATER, CYCLOPENTASILOXANE, CAPRYLYL METHICONE, BUTYLENE GLYCOL, CETYL PEG/PPG-10/1 DIMETHICONE, CYCLOHEXASILOXANE, NIACINAMIDE, ISOAMYL LAURATE, DIPHENYLSILOXY PHENYL TRIMETHICONE, C12-15 ALKYL ...
-
PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information