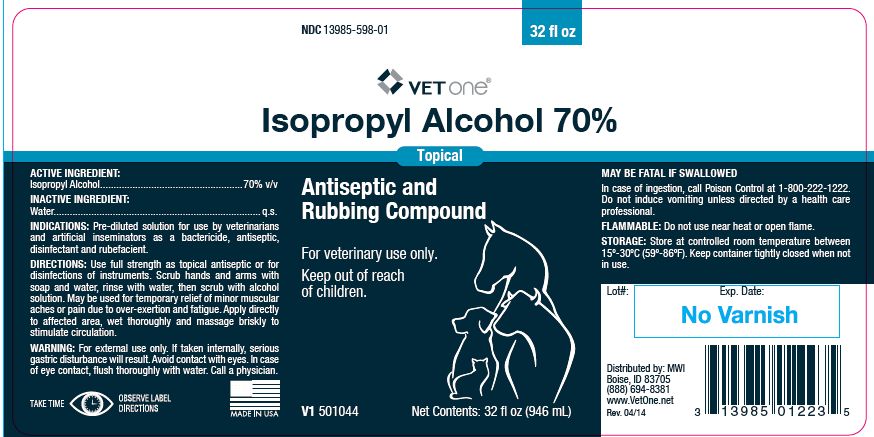
Label: ISOPROPYL ALCOHOL 70%- isopropyl alcohol liquid
- NDC Code(s): 13985-598-01
- Packager: MWI/Vet One
- Category: OTC ANIMAL DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 29, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- ACTIVE INGREDIENT:
- INACTIVE INGREDIENT:
- INDICATIONS:
-
DIRECTIONS:
Use full strength as topical antiseptic or for disinfections of instruments. Scrub hands and arms with soap and water, rinse with water, then scrub with alcohol solution. May be used for temporary relief of minor muscular aches or pain due to over-exertion and fatigue. Apply directly to affected area, wet thoroughly and massage briskly to stimulate circulation.
- WARNING:
- WARNINGS
- ADVERSE REACTIONS
- FLAMMABLE:
- STORAGE:
- Net Contents:
- INFORMATION FOR OWNERS/CAREGIVERS
- 32 fl oz (946 mL)
- 16 X 32 fl oz (946 mL) - Master Case Label
-
INGREDIENTS AND APPEARANCE
ISOPROPYL ALCOHOL 70%
isopropyl alcohol liquidProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:13985-598 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 557.25 g in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-598-01 16 in 1 CASE 1 4 in 1 BOX 1 0.946 L in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/15/2014 Labeler - MWI/Vet One (019926120) Establishment Name Address ID/FEI Business Operations FIRST PRIORITY INCORPORATED 179925722 manufacture, label, api manufacture