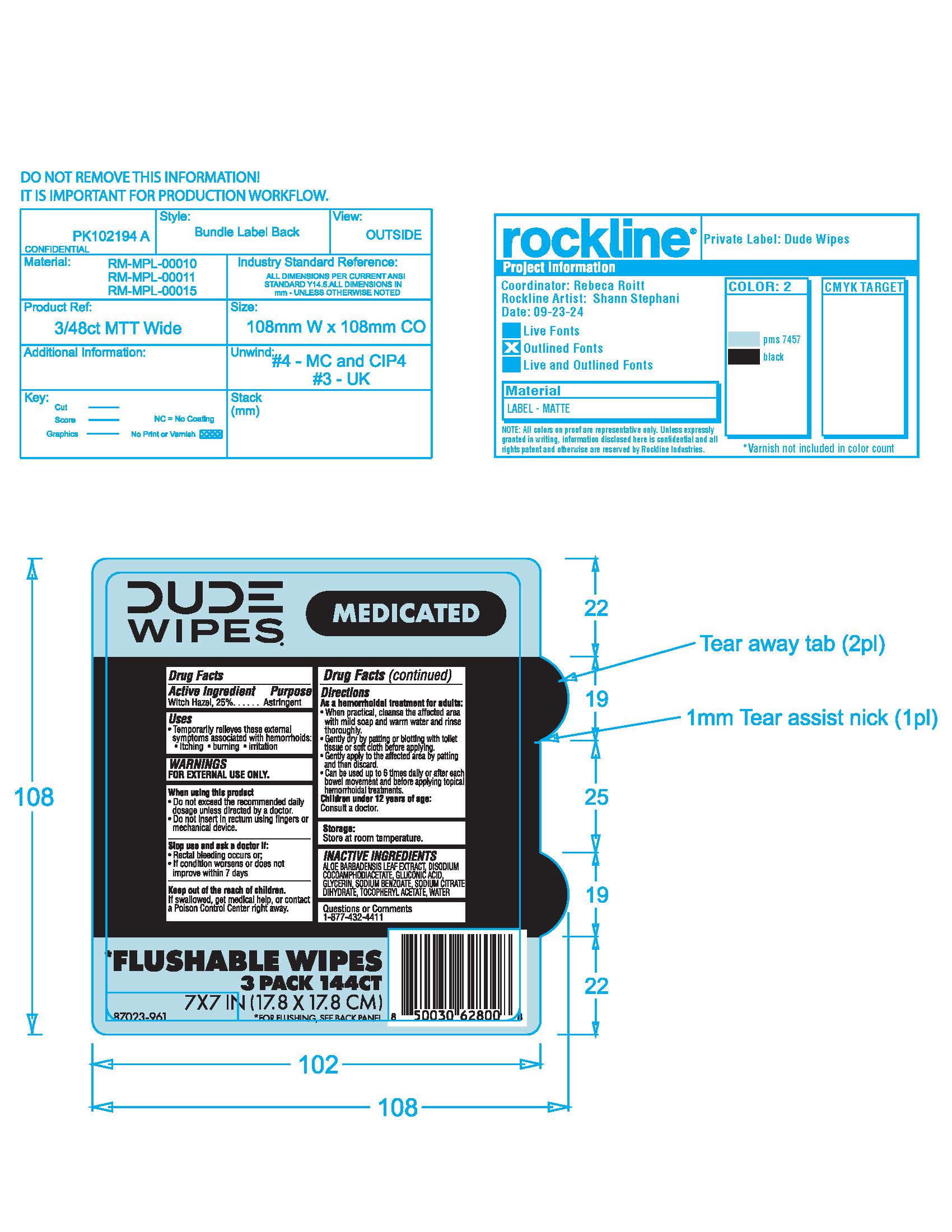
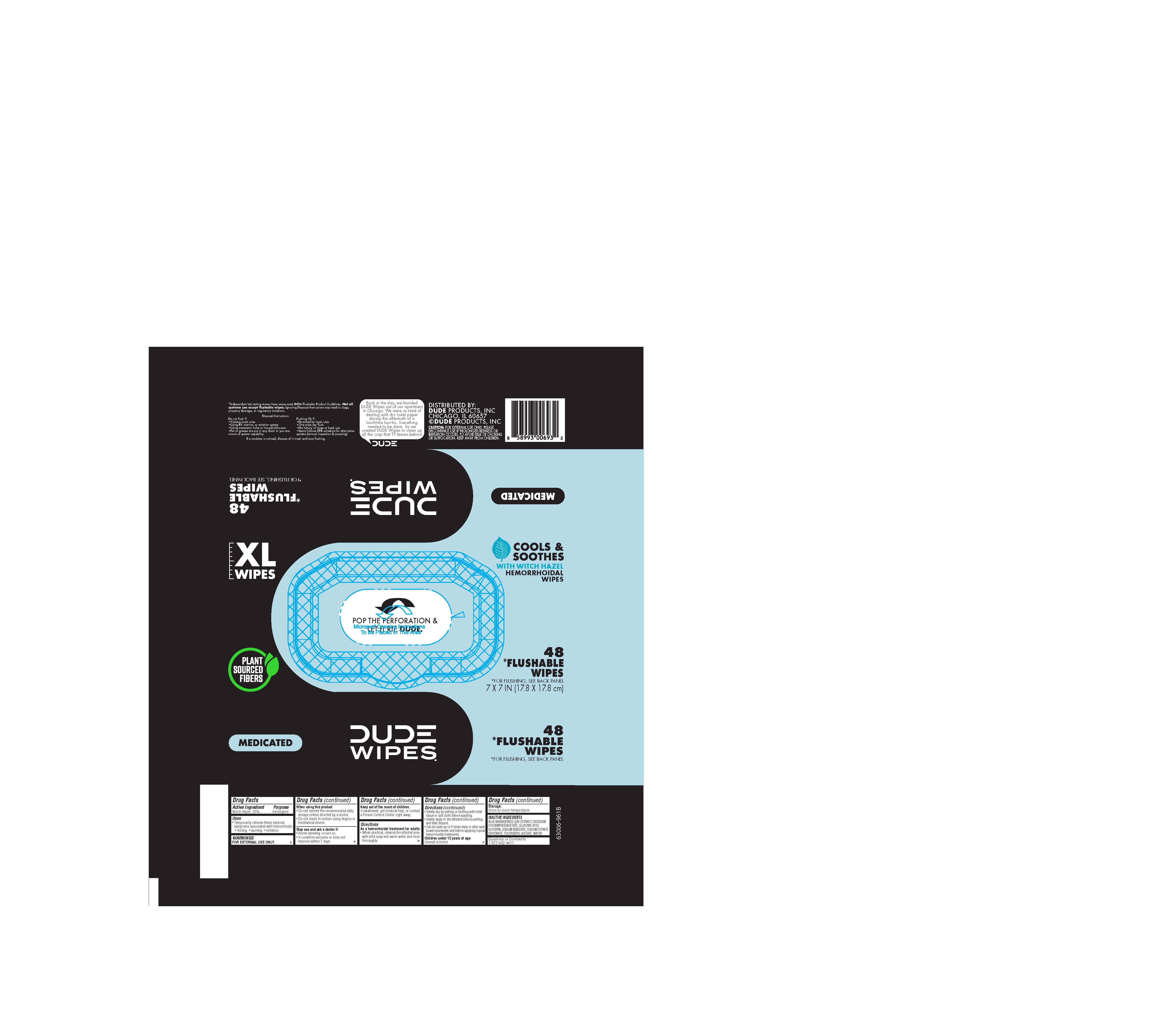
Label: DUDE HEMORRHOIDAL WIPES- witch hazel cloth
- NDC Code(s): 83731-055-03, 83731-055-48
- Packager: Dude Products
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 15, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
-
Directions
As a hemorrhoidal treatment for adults:
• When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly.
• Gently dry by patting or blotting with toilet tissue or soft cloth before applying.
• Gently apply to the affected area by patting and then discard.
Children under 12 years of age: Consult a doctor - Dosage
- Storage
- Inactive ingredients
- Questions or Comments
- Package Label
-
INGREDIENTS AND APPEARANCE
DUDE HEMORRHOIDAL WIPES
witch hazel clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83731-055 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WITCH HAZEL (UNII: 101I4J0U34) (WITCH HAZEL - UNII:101I4J0U34) WITCH HAZEL 1.5 g Inactive Ingredients Ingredient Name Strength GLUCONIC ACID (UNII: R4R8J0Q44B) ALOE VERA LEAF (UNII: ZY81Z83H0X) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) SODIUM BENZOATE (UNII: OJ245FE5EU) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83731-055-48 48 in 1 PACKAGE; Type 0: Not a Combination Product 01/14/2024 2 NDC:83731-055-03 3 in 1 PACKAGE 12/31/2024 2 NDC:83731-055-48 48 in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 01/14/2024 Labeler - Dude Products (078446360)