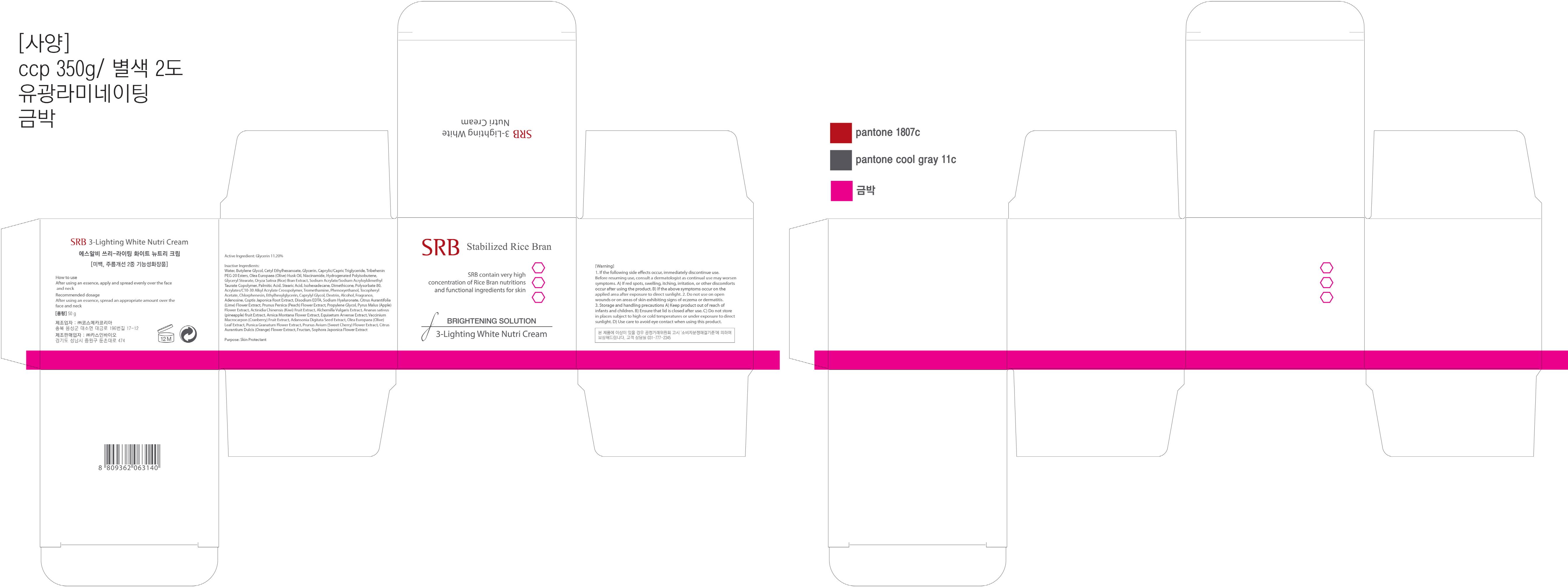
Label: SRB 3 LIGHTING WHITE NUTRI- glycerin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 70218-010-01 - Packager: CASINBIO CO., LTD.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 12, 2015
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive Ingredients: Water, Butylene Glycol, Cetyl Ethylhexanoate, Caprylic/Capric Triglyceride, Tribehenin PEG-20 Esters, Olea Europaea (Olive) Husk Oil, Niacinamide, Hydrogenated Polyisobutene, Glyceryl Stearate, Oryza Sativa (Rice) Bran Extract, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Palmitic Acid, Stearic Acid, Isohexadecane, Dimethicone, Polysorbate 80, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Tromethamine, Phenoxyethanol, Tocopheryl Acetate, Chlorphenesin, Ethylhexylglycerin, Caprylyl Glycol, Dextrin, Alcohol, Fragrance, Adenosine, Coptis Japonica Root Extract, Disodium EDTA, Sodium Hyaluronate, Citrus Aurantifolia (Lime) Flower Extract, Prunus Persica (Peach) Flower Extract, Propylene Glycol, Pyrus Malus (Apple) Flower Extract, Actinidia Chinensis (Kiwi) Fruit Extract, Alchemilla Vulgaris Extract, Ananas sativus (pineapple) fruit Extract, Arnica Montana Flower Extract, Equisetum Arvense Extract, Vaccinium Macrocarpon (Cranberry) Fruit Extract, Adansonia Digitata Seed Extract, Olea Europaea (Olive) Leaf Extract, Punica Granatum Flower Extract, Prunus Avium (Sweet Cherry) Flower Extract, Citrus Aurantium Dulcis (Orange) Flower Extract, Fructan, Sophora Japonica Flower Extract
- PURPOSE
-
WARNINGS
Warnings: 1. If the following side effects occur, immediately discontinue use. Before resuming use, consult a dermatologist as continual use may worsen symptoms. A) If red spots, swelling, itching, irritation, or other discomforts occur after using the product. B) If the above symptoms occur on the applied area after exposure to direct sunlight. 2. Do not use on open wounds or on areas of skin exhibiting signs of eczema or dermatitis. 3. Storage and handling precautions A) Keep product out of reach of infants and children. B) Ensure that lid is closed after use. C) Do not store in places subject to high or cold temperatures or under exposure to direct sunlight. D) Use care to avoid eye contact when using this product.
- KEEP OUT OF REACH OF CHILDREN
- How to use
- Recommended dosage
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SRB 3 LIGHTING WHITE NUTRI
glycerin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70218-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Glycerin (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) Glycerin 5.6 g in 50 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Butylene Glycol (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70218-010-01 50 g in 1 CARTON; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/01/2015 Labeler - CASINBIO CO., LTD. (557830673) Registrant - CASINBIO CO., LTD. (557830673) Establishment Name Address ID/FEI Business Operations CASINBIO CO., LTD. 557830673 manufacture(70218-010)