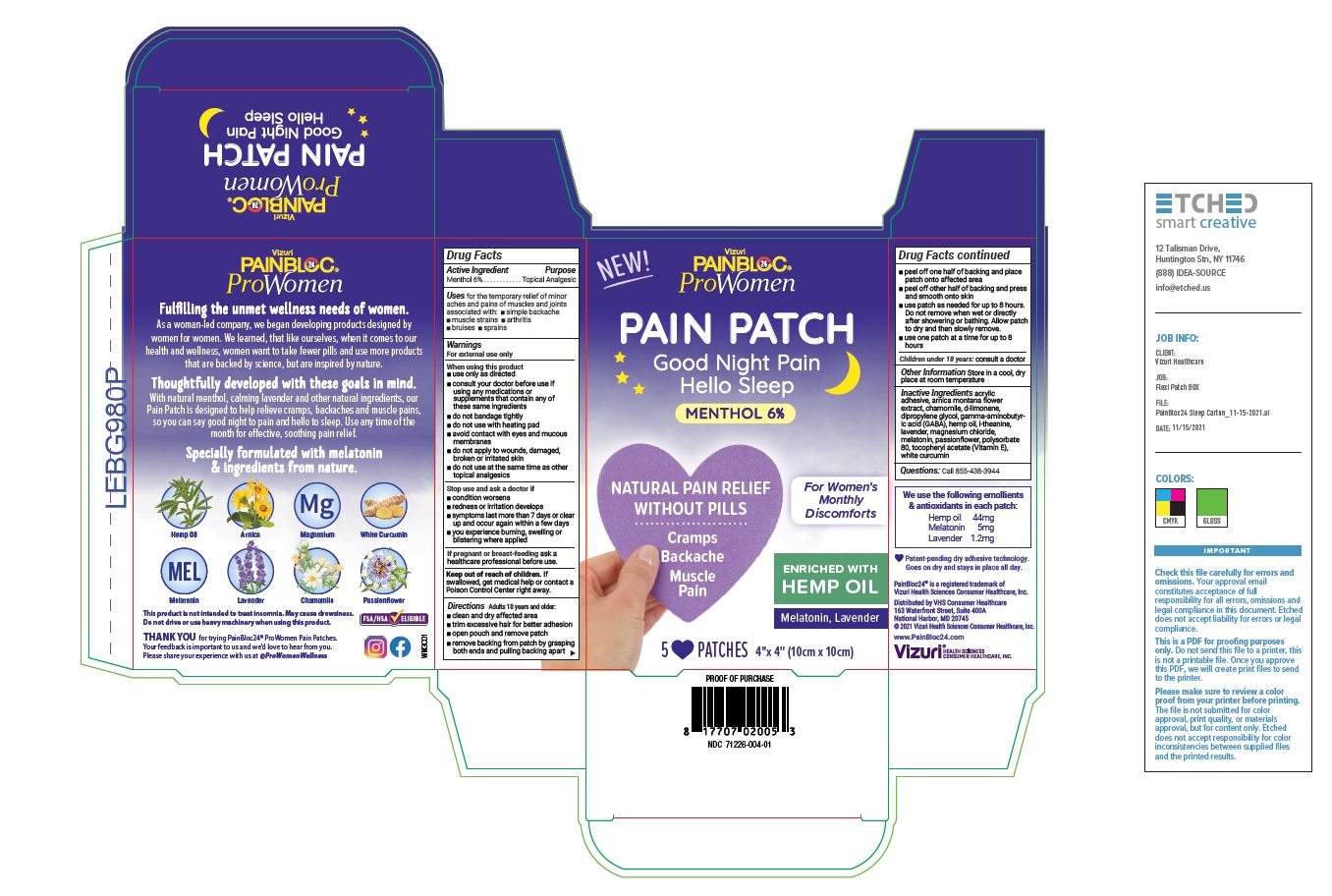
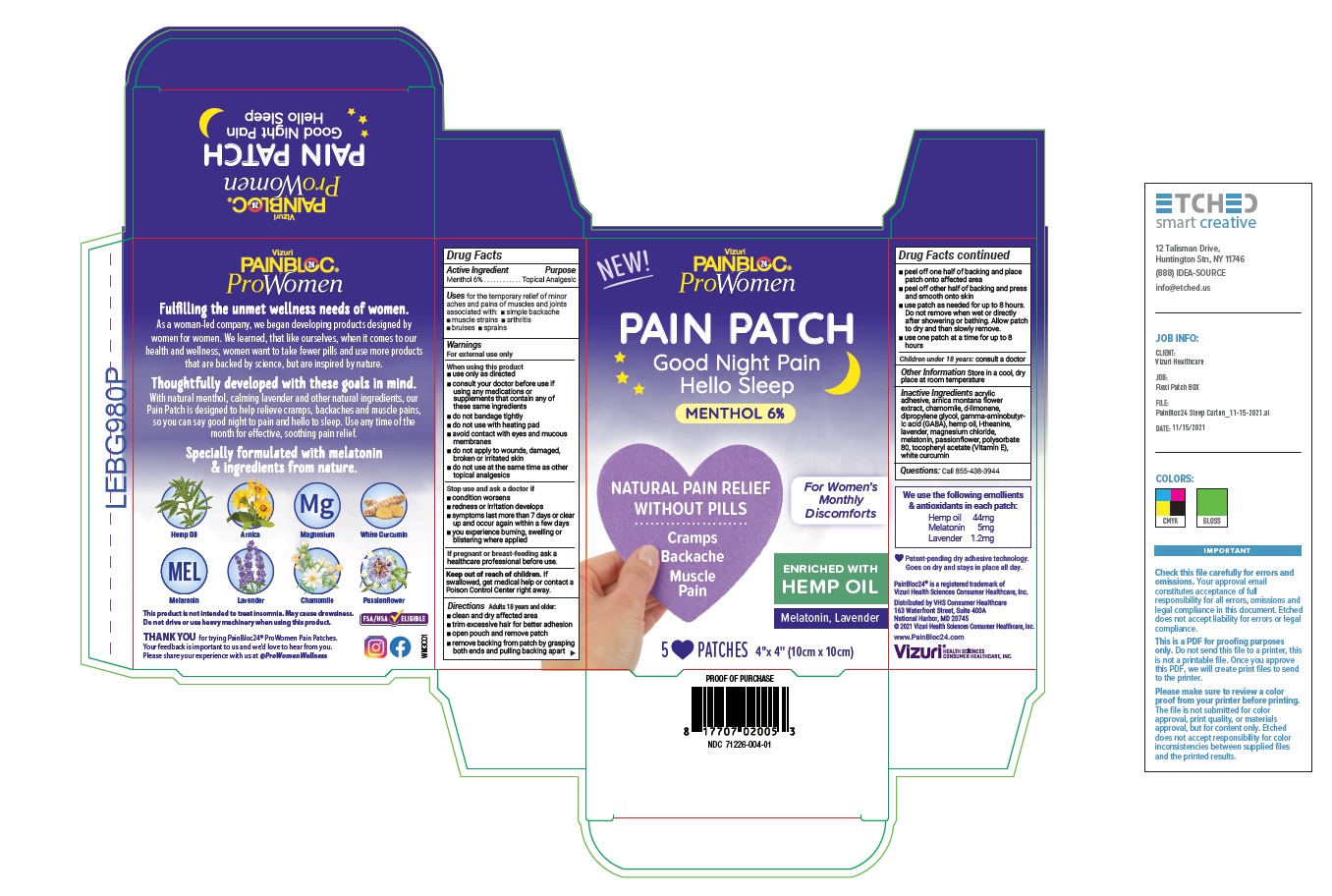
Label: PAINBLOC24 PROWOMEN WOMENS DISCOMFORTS MENTHOL PAIN AND SLEEP PATCH- menthol 6% patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 71226-004-01 - Packager: VIZURI HEALTH SCIENCES LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 10, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- use only as directed
- consult your doctor before use if using any medications or supplements that contain any of these same ingredients
- do not bandage tightly
- do not use with heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds, damaged, broken or irritated skin
- do not use at the same time as other topical analgesics
-
Directions
Adults over 18 years:
- clean and dry affected area
- trim excessive hair for better adhesion
- open pouch and remove patch
- remove backing from patch by grasping both ends and pulling backing apart
- peel off one half of backing and place patch onto affected area
- peel off other half of backing and press and smooth onto skin
- use patch as needed for up to 8 hours. Do not remove when wet or directly after showering or bathing. Allow patch to dry and then slowly remove.
- use one patch at a time for up to 8 hours
Children under 18 years: consult a doctor
- Inactive Ingredients
- Questions
- Other Information
- Product label
-
INGREDIENTS AND APPEARANCE
PAINBLOC24 PROWOMEN WOMENS DISCOMFORTS MENTHOL PAIN AND SLEEP PATCH
menthol 6% patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71226-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 6 g Inactive Ingredients Ingredient Name Strength ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) CHAMOMILE (UNII: FGL3685T2X) LIMONENE, (+)- (UNII: GFD7C86Q1W) DIPROPYLENE GLYCOL (UNII: E107L85C40) .GAMMA.-AMINOBUTYRIC ACID (UNII: 2ACZ6IPC6I) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) THEANINE (UNII: 8021PR16QO) LAVANDULA ANGUSTIFOLIA SUBSP. ANGUSTIFOLIA FLOWER (UNII: 19AH1RAF4M) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) MELATONIN (UNII: JL5DK93RCL) PASSIFLORA INCARNATA FLOWER (UNII: K8F3G29S6Z) POLYSORBATE 80 (UNII: 6OZP39ZG8H) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CURCUMIN (UNII: IT942ZTH98) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71226-004-01 1 in 1 CARTON 01/25/2022 1 5 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/25/2022 Labeler - VIZURI HEALTH SCIENCES LLC (052129499) Establishment Name Address ID/FEI Business Operations Shawsheen Rubber Co. 001051275 manufacture(71226-004)