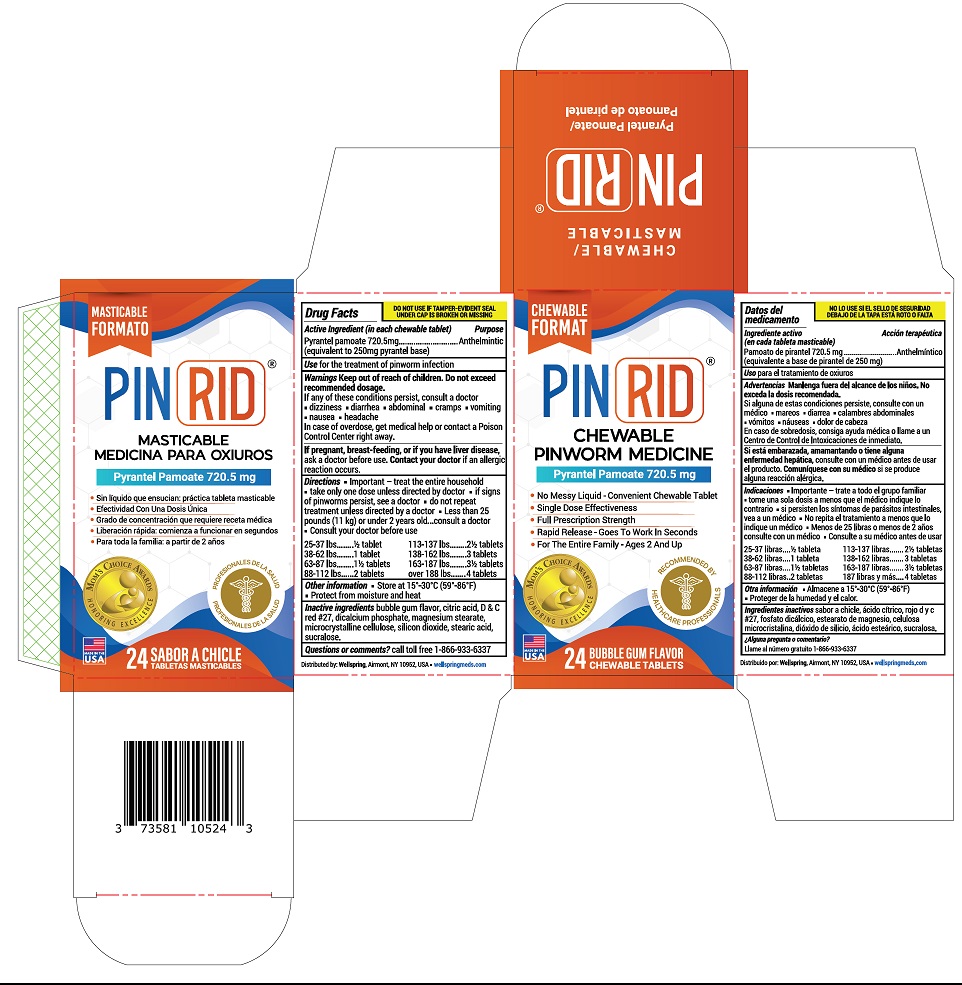
Label: PINRID- pyrantel pamoate tablet
- NDC Code(s): 73581-111-12, 73581-111-24
- Packager: YYBA CORP
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 1, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient (in each chewable tablet)
- Purpose
- Use
- Warnings
-
Directions
- Important- treat the entire household
- take only one dose unless directed by doctor
- if signs of pinworms persist, see a doctor
- do not repeat treatment unless directed by a doctor
- less than 25 pounds (11 kg) or under 2 years old, consult a doctor
- Consult your doctor before use
25-37 lb ½ tablet 38-62 lbs 1 tablets 63-87 lbs 1½ tablets 88-112 lbs 2 tablets 113-137 lbs 2½ tablets 138-162 lbs 3 tablets 163-187 lbs 3½ tablets over 188 lbs 4 tablets - Other information:
- Intactive ingredients
- Questions or comments:
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PINRID
pyrantel pamoate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73581-111 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 250 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) D&C RED NO. 27 (UNII: 2LRS185U6K) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color pink Score 2 pieces Shape ROUND Size 16mm Flavor Imprint Code PR1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73581-111-12 12 in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2024 2 NDC:73581-111-24 24 in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M024 08/01/2024 Labeler - YYBA CORP (006339772)