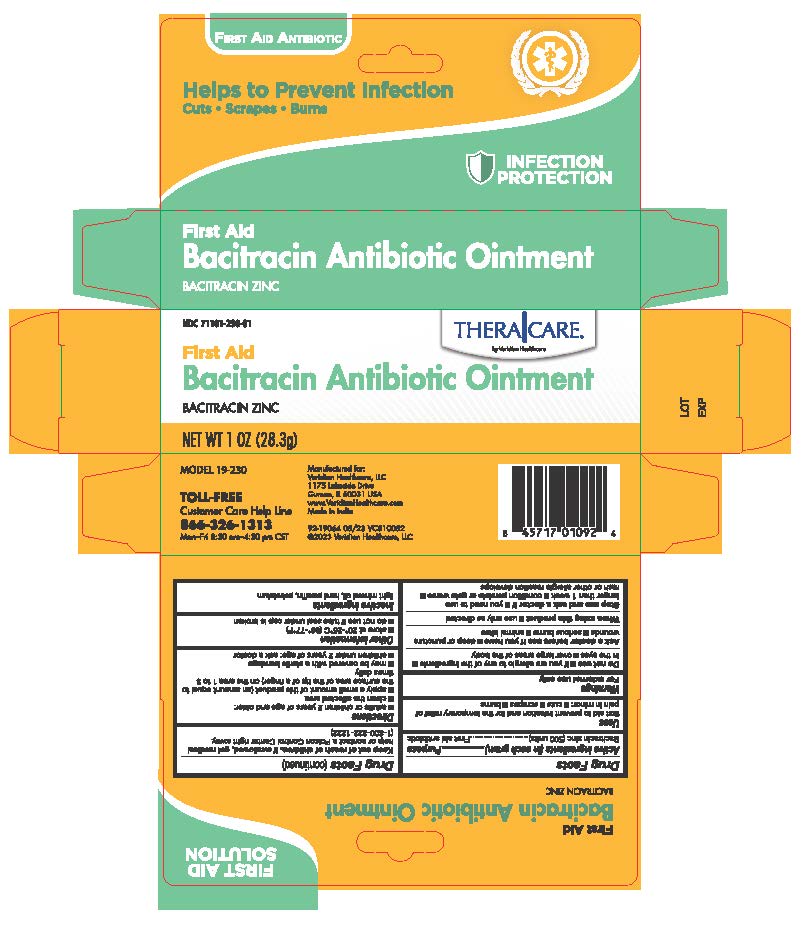
Label: THERACARE BACITRACIN ZINC- first aid antibiotic ointment
- NDC Code(s): 71101-230-01
- Packager: Veridian Healthcare
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 18, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions and comments?
- Label
-
INGREDIENTS AND APPEARANCE
THERACARE BACITRACIN ZINC
first aid antibiotic ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71101-230 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 500 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength PARAFFIN (UNII: I9O0E3H2ZE) PETROLATUM (UNII: 4T6H12BN9U) LIGHT MINERAL OIL (UNII: N6K5787QVP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71101-230-01 1 in 1 BOX 05/01/2023 1 28.3 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 05/01/2023 Labeler - Veridian Healthcare (830437997) Establishment Name Address ID/FEI Business Operations ANICARE PHARMACEUTICALS PRIVATE LIMITED 916837425 manufacture(71101-230)