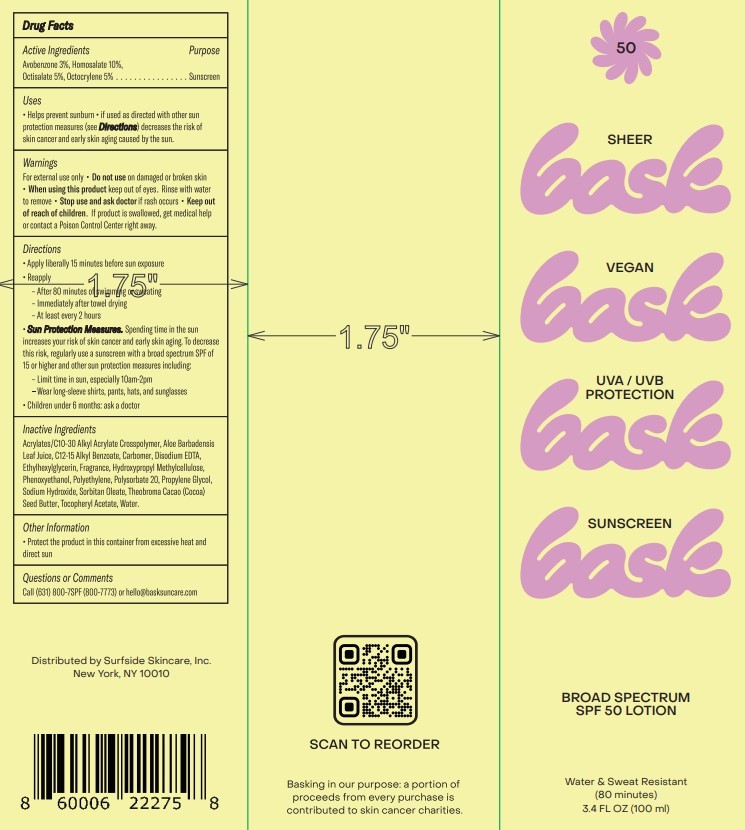
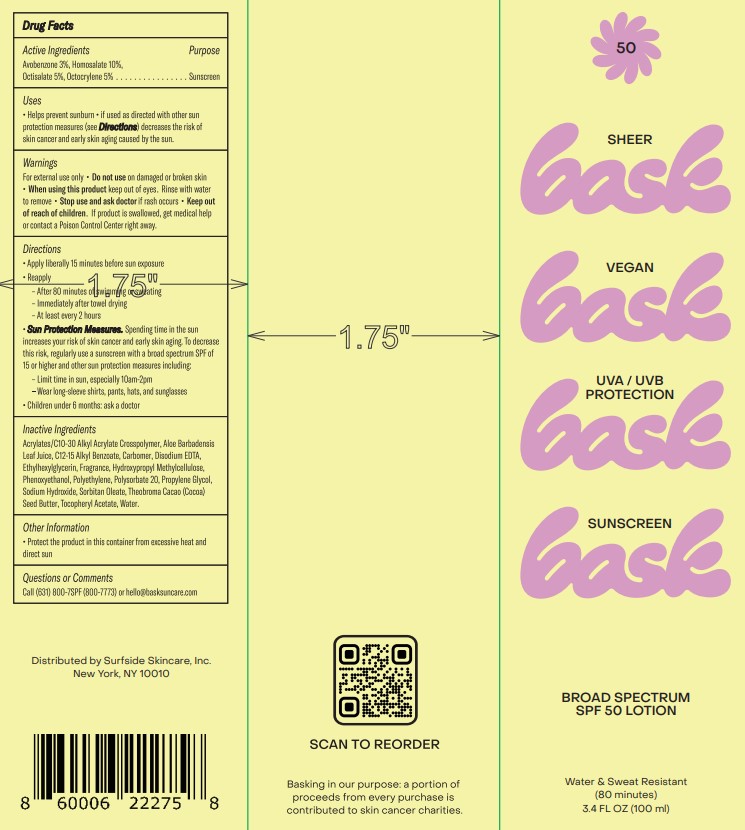
Label: BASK BROAD SPECTRUM SPF 50- avobenzone, homosalate, octisalate, octocrylene lotion
- NDC Code(s): 58443-0615-3
- Packager: Prime Enterprises Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 9, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
• Apply liberally 15 minutes before sun exposure
• Reapply
• After 80 minutes of swimming or sweating
• Immediately after towel drying
• At least every 2 hours
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
• Limit time in the sun, especially from 10 am – 2 pm
• Wear long-sleeve shirts, pants, hats, and sunglasses
• Children under 6 months: Ask a doctor.
-
Inactive Ingredients
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, C12-15 Alkyl Benzoate, Carbomer, Disodium EDTA, Ethylhexylglycerin, Fragrance, Hydroxypropyl Methylcellulose, Phenoxyethanol, Polyethylene, Polysorbate 20, Propylene Glycol, Sodium Hydroxide, Sorbitan Oleate, Theobroma Cacao (Cocoa) Seed Butter, Tocopheryl Acetate, Water
- Other Information
- Questions or Comments
- BASK Sunscreen Lotion Broad Spectrum SPF 50
-
INGREDIENTS AND APPEARANCE
BASK BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58443-0615 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 50 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 99 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) POLYSORBATE 20 (UNII: 7T1F30V5YH) COCOA BUTTER (UNII: 512OYT1CRR) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM (UNII: 7FLD91C86K) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CARBOMER 980 (UNII: 4Q93RCW27E) Product Characteristics Color white (White to Off-White) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58443-0615-3 100 mL in 1 TUBE; Type 0: Not a Combination Product 01/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 01/30/2019 Labeler - Prime Enterprises Inc. (101946028) Registrant - Prime Enterprises Inc. (101946028) Establishment Name Address ID/FEI Business Operations Prime Enterprises Inc. 101946028 pack(58443-0615) , manufacture(58443-0615) , label(58443-0615) , analysis(58443-0615)