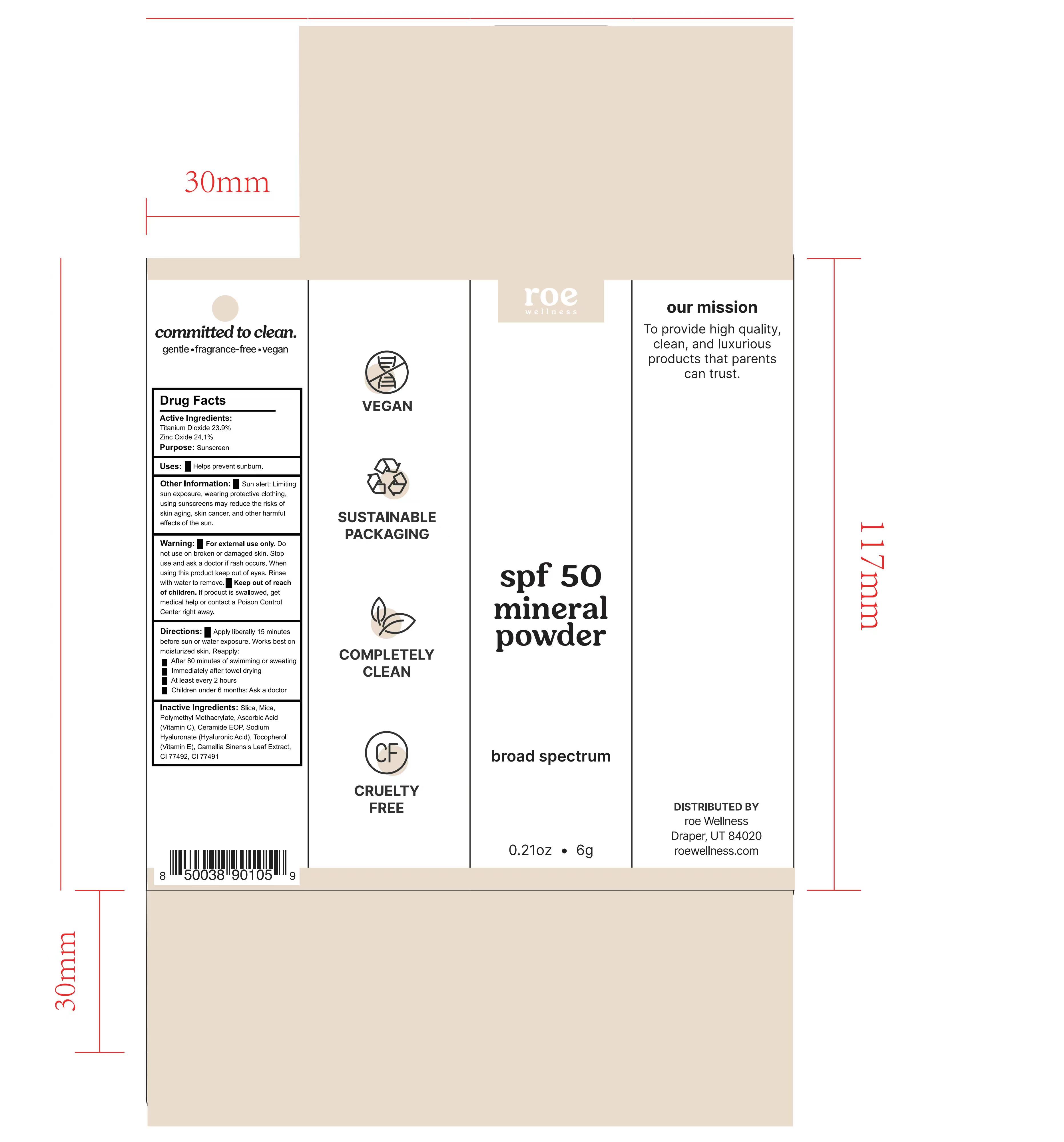
Label: ROE WELLNESS SPF 50 MINERAL POWDER- spf 50 mineral powder powder
- NDC Code(s): 82723-003-01
- Packager: Aopline Health Industry Technology (Guangzhou) Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 18, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Keep out of reach of children.
- Stop use
- WHEN USING
- Do not use
- Package Label
-
INGREDIENTS AND APPEARANCE
ROE WELLNESS SPF 50 MINERAL POWDER
spf 50 mineral powder powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82723-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 23.9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 24.1 g in 100 g Inactive Ingredients Ingredient Name Strength CERAMIDE NP (UNII: 4370DF050B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYL ACRYLATE AND METHYL METHACRYLATE COPOLYMER DISPERSION (2:1; 600000 MW 30% AQUEOUS) (UNII: 3EW60Z566Z) .ALPHA.-TOCOPHEROL CALCIUM SUCCINATE, DL- (UNII: H5A1374A6R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82723-003-01 6 g in 1 BOX; Type 0: Not a Combination Product 05/07/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/07/2022 Labeler - Aopline Health Industry Technology (Guangzhou) Co., Ltd. (715076108) Establishment Name Address ID/FEI Business Operations Aopline Health Industry Technology (Guangzhou) Co., Ltd. 715076108 manufacture(82723-003)