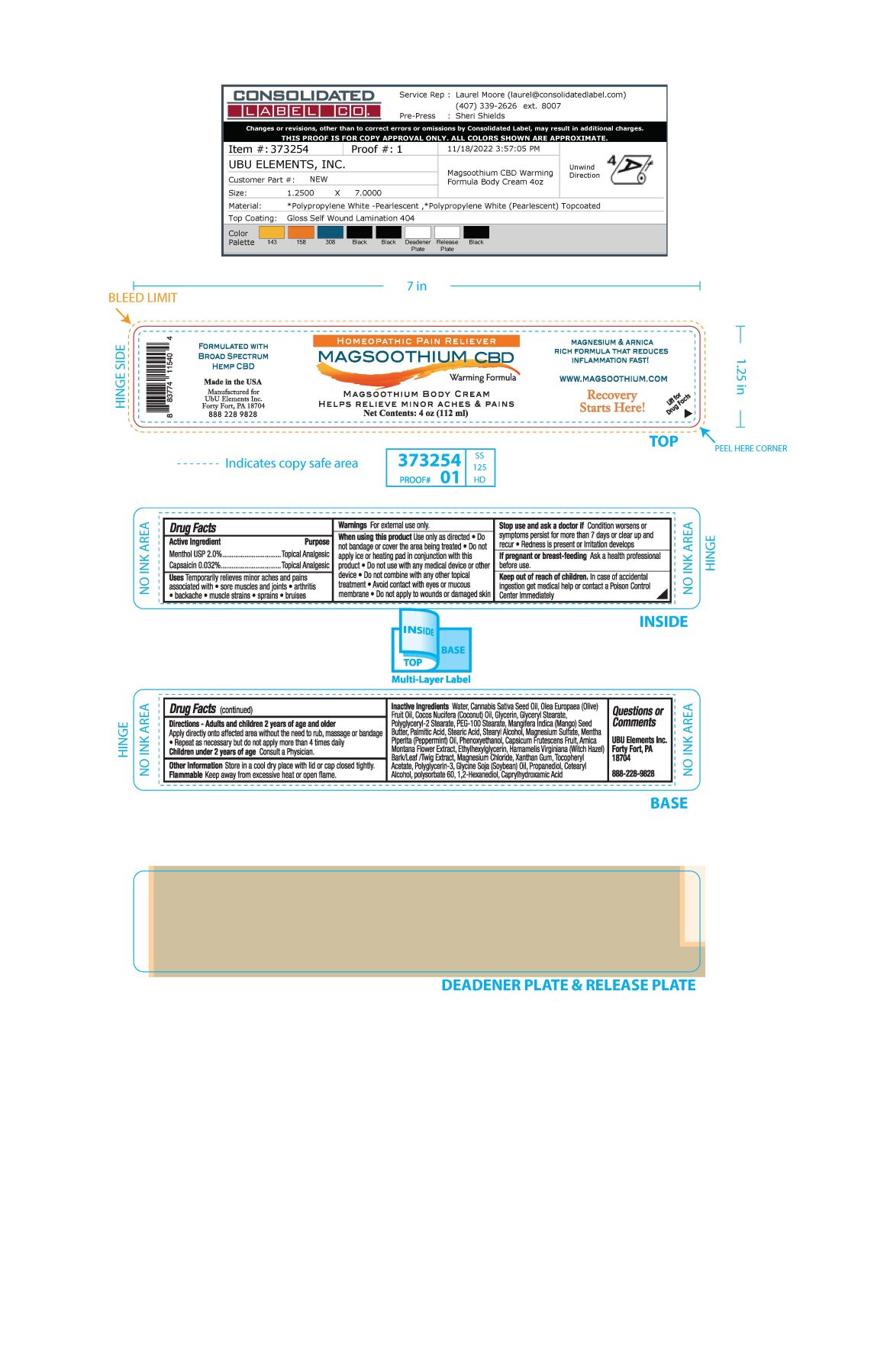
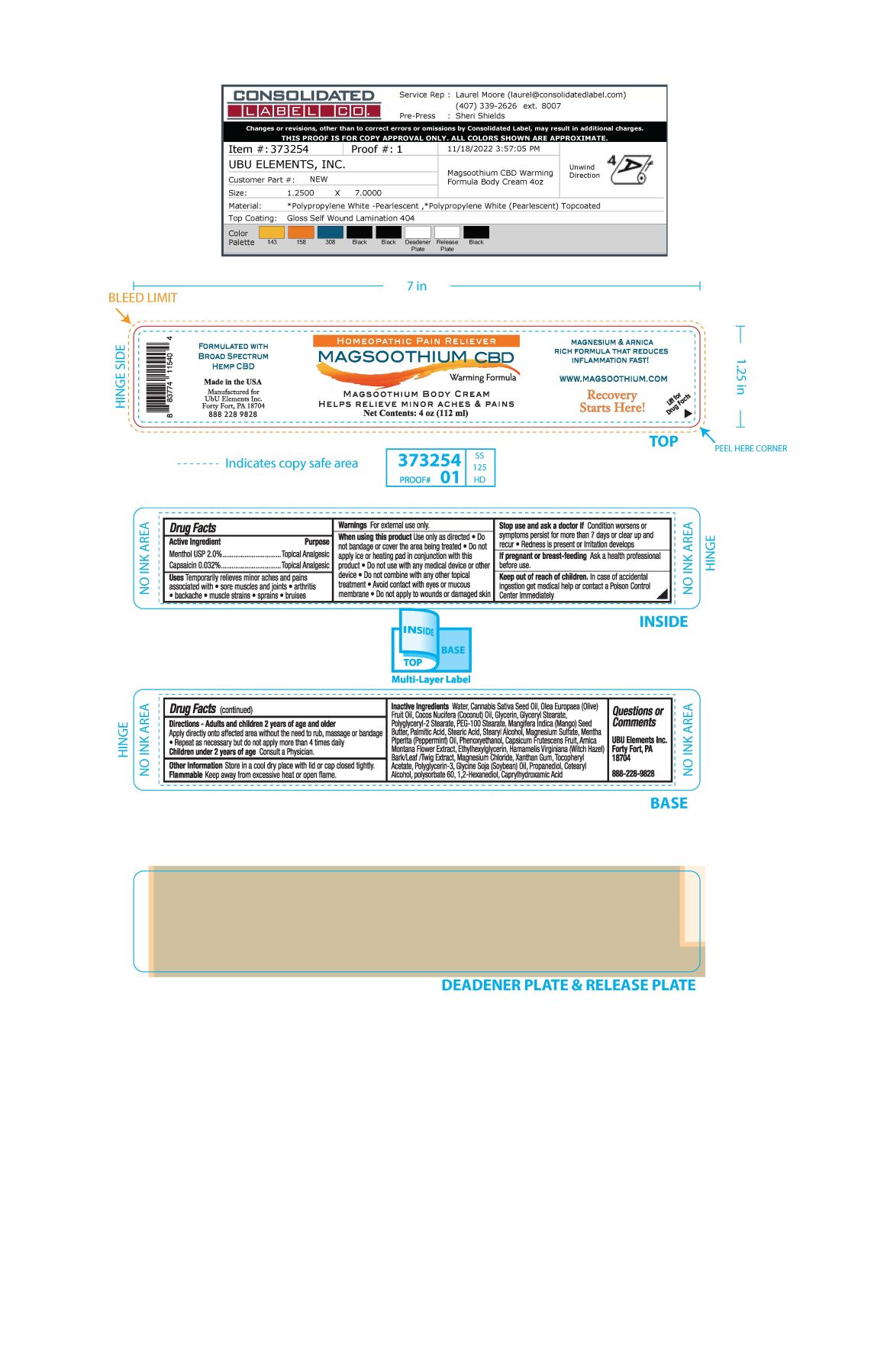
Label: MAGSOOTHIUM BODY- menthol, capsaicin cream
- NDC Code(s): 83262-001-01
- Packager: Ubu/Elements, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 3, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Warnings For external use only
When using this product use only as directed
- Do not bandage or cover the area being treated
- Do not apply ice or heating pad in conjunction with this product.
- Do not use with any medical device or other device
- Do not combine with any other topical treatment
- Avoid contact with eyes or mucous membrane
- Do not apply to wounds or damaged skin
If pregnant or breast-feeding Ask a health professional before use
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients Water, Cannabis Sativa Seed Oil, Olea Europaea (Olive) Fruit Oil, Cocos Nucifera (Coconut) Oil, Glycerin, Glyceryl Stearate, Polyglyceryl-2 Stearate, PEG-100 Stearate, Mangifera indica (Mango) Seed Butter, Palmitic Acid, Stearic Acid, Stearyl Alcohol, Magnesium Sulfate, Mentha Piperita (Peppermint) Oil, Phenoxyethanol, Capsicum Frutescens Fruit, Arnica Montana Flower Extract, Ethylhexylglycerin, Hamamelis Virginiana (Witch Hazel) Bark/Leaf/Twig Extract, Magnesium Chloride, Xanthan Gum, Tocopheryl Acetate, Polyglycerin-3, Glycine Soja (Soybean) Oil, Propanediol, Cetearyl Acohol, Polysorbate 60, 1,2-Hexanediol, Caprylhydroxamic Acid
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MAGSOOTHIUM BODY
menthol, capsaicin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83262-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.032 g in 100 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 2 g in 100 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) PROPANEDIOL (UNII: 5965N8W85T) COCONUT OIL (UNII: Q9L0O73W7L) POLYSORBATE 60 (UNII: CAL22UVI4M) PALMITIC ACID (UNII: 2V16EO95H1) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) SOYBEAN OIL (UNII: 241ATL177A) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) OLIVE OIL (UNII: 6UYK2W1W1E) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) PEPPERMINT OIL (UNII: AV092KU4JH) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) POLYGLYCERYL-2 STEARATE (UNII: 253MC0P0YV) STEARIC ACID (UNII: 4ELV7Z65AP) PEG-100 STEARATE (UNII: YD01N1999R) TABASCO PEPPER (UNII: J1M3NA843L) XANTHAN GUM (UNII: TTV12P4NEE) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) HAMAMELIS VIRGINIANA LEAF (UNII: T07U1161SV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83262-001-01 112 mL in 1 JAR; Type 0: Not a Combination Product 02/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 02/01/2023 Labeler - Ubu/Elements, Inc. (117378343)