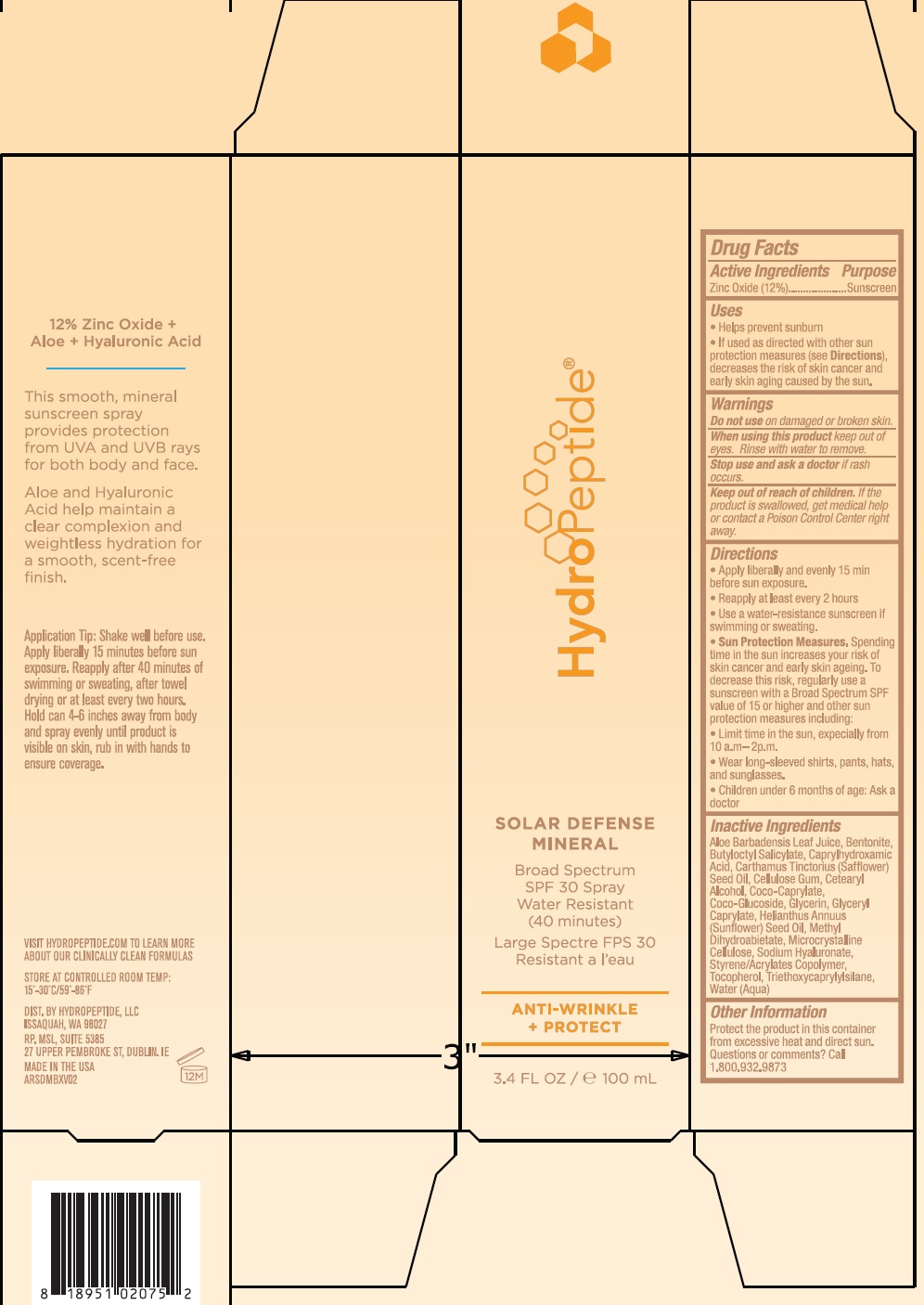
Label: HYDROPEPTIDE SOLAR DEFENSE MINERAL- zinc oxide cream
- NDC Code(s): 60934-013-00
- Packager: HydroPeptide LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 14, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
-
Directions
• Apply liberally and evenly 15 min before sun exposure. • Reapply at least every 2 hours • Use a water-resistant sunscreen if swimming or sweating. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • Limit time in the sun, especially from 10 a.m. - 2 p.m. • Wear long-sleeved shirts, pants, hats, and sunglasses. • Children under 6 months of age: Ask a doctor
• Sun Protection Measures. -
Inactive Ingredients
Aloe Barbadensis Leaf Juice, Bentonite, Butyloctyl Salicylate, Caprylhydroxamic Acid, Carthamus Tinctorius (Safflower) Seed Oil, Cellulose Gum, Cetearyl Alcohol, Coco-Caprylate, Coco-Glucoside, Glycerin, Glyceryl Caprylate, Helianthus Annuus (Sunflower) Seed Oil, Methyl Dihydroabietate, Microcrystalline Cellulose, Sodium Hyaluronate, Styrene/Acrylates Copolymer, Tocopherol, Triethoxycaprylylsilane, Water (Aqua)
- Other Information
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
HYDROPEPTIDE SOLAR DEFENSE MINERAL
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60934-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) BENTONITE (UNII: A3N5ZCN45C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) SAFFLOWER OIL (UNII: 65UEH262IS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) COCO-CAPRYLATE (UNII: 4828G836N6) COCO GLUCOSIDE (UNII: ICS790225B) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOCAPRYLATE (UNII: TM2TZD4G4A) SUNFLOWER OIL (UNII: 3W1JG795YI) METHYL DIHYDROABIETATE (UNII: 7666FJ0J9F) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TOCOPHEROL (UNII: R0ZB2556P8) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60934-013-00 1 in 1 CARTON 01/01/2021 1 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2021 Labeler - HydroPeptide LLC (006297465)