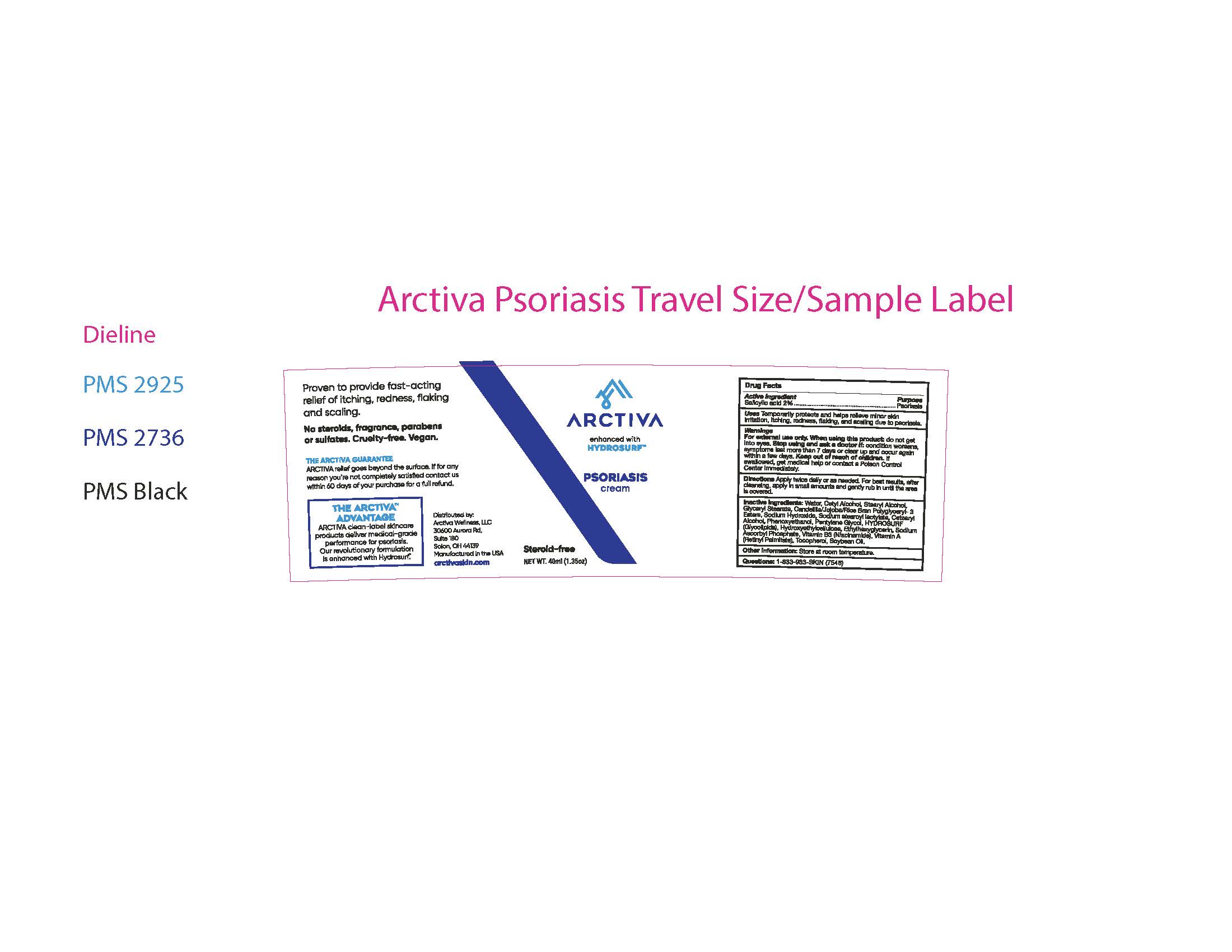
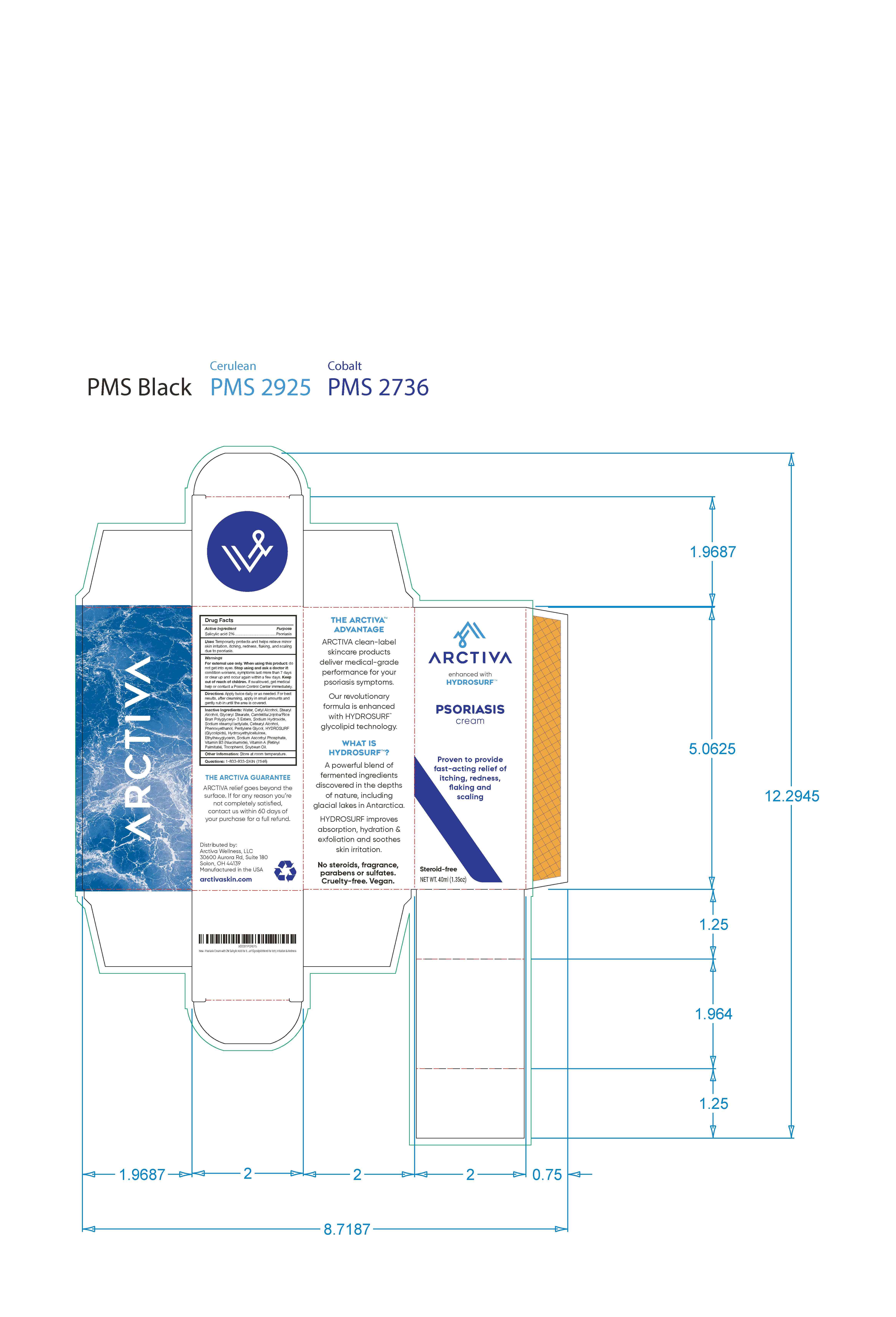
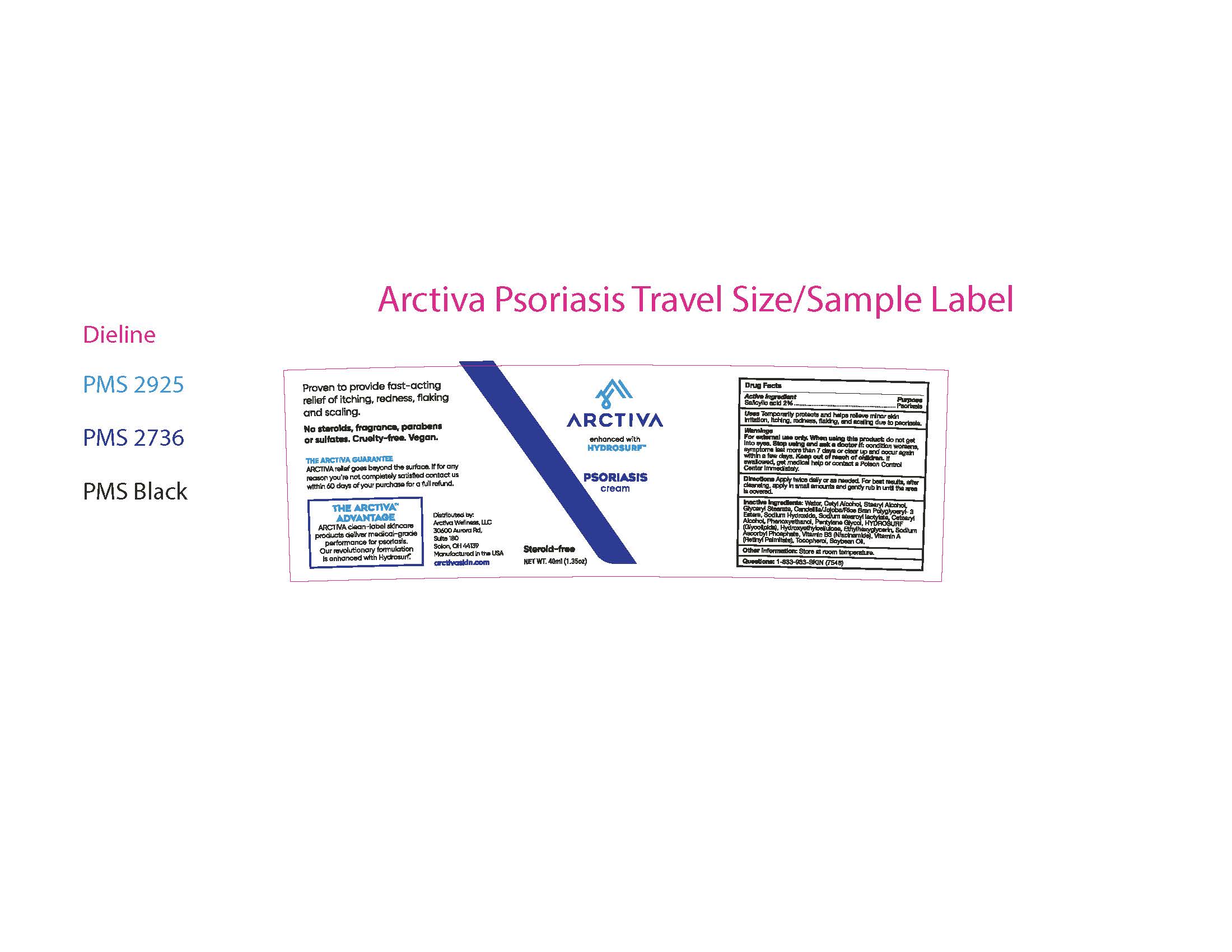
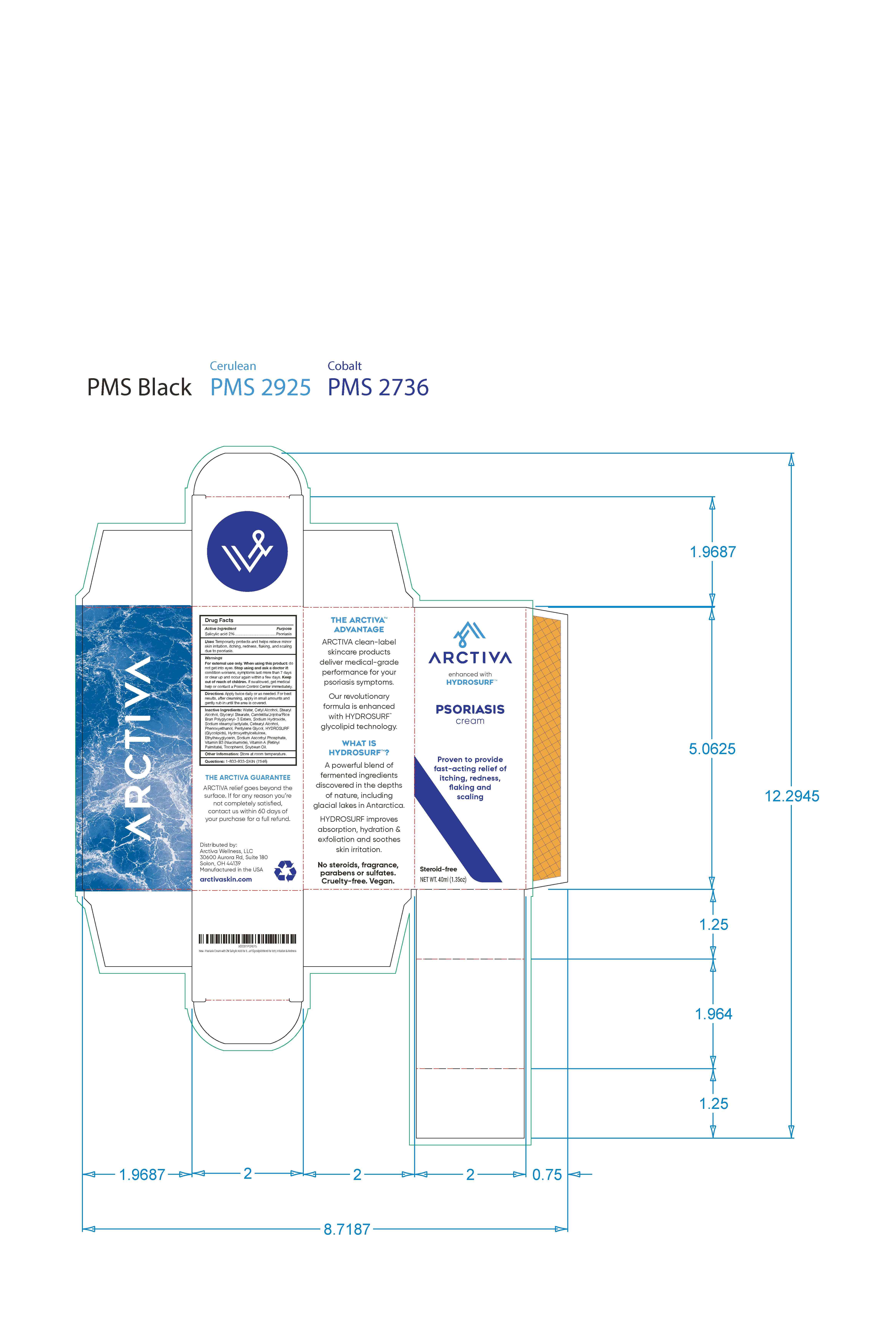
Label: ARCTIVA PSORIASIS CREAM- psoriasis cream cream
- NDC Code(s): 58418-822-40, 58418-822-44, 58418-822-45, 58418-822-99
- Packager: Tropical Enterprises International, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 10, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients: Water, Cetyl Alcohol,
Stearyl Alcohol, Glyceryl Stearate,
Candelilla/Jojoba/Rice Bran Polyglyceryl- 3
Esters, Sodium Hydroxide, Sodium stearoyl
lactylate, Cetearyl Alcohol, Phenoxyethanol,
Pentylene Glycol, Hydrosurf (Glycolipids),
Hydroxyethylcellulose, Ethylhexyglycerin,
Sodium Ascorbyl Phosphate, Vitamin B3
(Niacinamide), Vitamin A (Retinyl Palmitate),
Tocopherol, Soybean Oil.
- STORAGE AND HANDLING
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- 130ml
- 40ml label and outer carton
-
INGREDIENTS AND APPEARANCE
ARCTIVA PSORIASIS CREAM
psoriasis cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58418-822 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2.6 g in 130 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETYL ALCOHOL (UNII: 936JST6JCN) PENTYLENE GLYCOL (UNII: 50C1307PZG) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM STEAROYL LACTYLATE (UNII: IN99IT31LN) NIACINAMIDE (UNII: 25X51I8RD4) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) CANDELILLA WAX (UNII: WL0328HX19) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) RICE BRAN (UNII: R60QEP13IC) LIPID A 504 (UNII: Q2VF73396U) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROXYETHYL CELLULOSE (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) Product Characteristics Color white (lotion) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58418-822-99 1 in 1 CARTON 02/01/2023 1 NDC:58418-822-45 130 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:58418-822-44 1 in 1 CARTON 10/02/2023 2 NDC:58418-822-40 40 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 02/01/2023 Labeler - Tropical Enterprises International, Inc. (091986179)