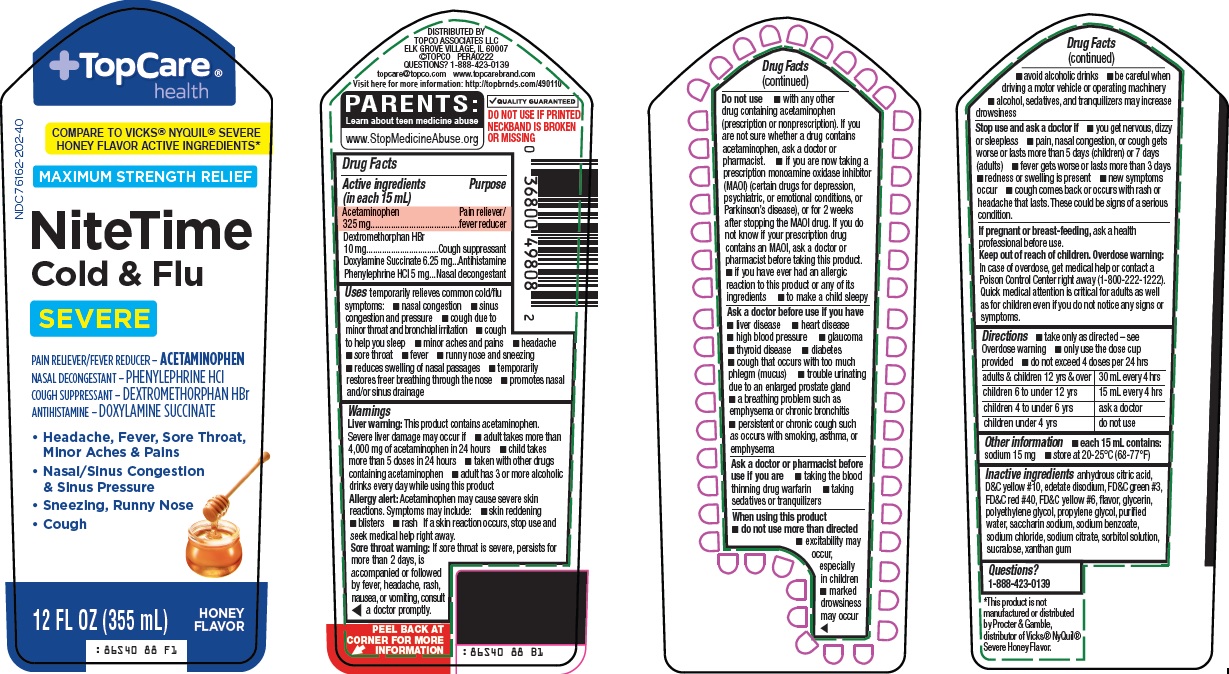
Label: TOPCARE NITE TIME COLD AND FLU- acetaminophen, dextromethorphan hydrobromide, doxylamine succinate, phenylephrine hydrochloride solution
- NDC Code(s): 76162-202-40
- Packager: Topco Associates LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 11, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 15 mL)
- Purpose
-
Uses
temporarily relieves common cold/flu symptoms:
- •
- nasal congestion
- •
- sinus congestion and pressure
- •
- cough due to minor throat and bronchial irritation
- •
- cough to help you sleep
- •
- minor aches and pains
- •
- headache
- •
- fever
- •
- sore throat
- •
- runny nose and sneezing
- •
- reduces swelling of nasal passages
- •
- temporarily restores freer breathing through the nose
- •
- promotes nasal and/or sinus drainage
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if
- •
- adult takes more than 4,000 mg of acetaminophen in 24 hours
- •
- child takes more than 5 doses in 24 hours
- •
- taken with other drugs containing acetaminophen
- •
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- •
- to make a child sleepy
- •
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- •
- liver disease
- •
- heart disease
- •
- glaucoma
- •
- thyroid disease
- •
- diabetes
- •
- high blood pressure
- •
- trouble urinating due to an enlarged prostate gland
- •
- cough that occurs with too much phlegm (mucus)
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
Ask a doctor or pharmacist before use if you are
- •
- taking the blood thinning drug warfarin
- •
- taking sedatives or tranquilizers
When using this product
- •
- do not use more than directed
- •
- excitability may occur, especially in children
- •
- marked drowsiness may occur
- •
- avoid alcoholic drinks
- •
- be careful when driving a motor vehicle or operating machinery
- •
- alcohol, sedatives, and tranquilizers may increase drowsiness
Stop use and ask a doctor if
- •
- you get nervous, dizzy or sleepless
- •
- pain, nasal congestion, or cough gets worse or lasts more than 5 days (children) or 7 days (adults)
- •
- fever gets worse or lasts more than 3 days
- •
- redness or swelling is present
- •
- new symptoms occur
- •
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
- Directions
- Other information
- Inactive ingredients
- Questions?
-
Package/Label Principal Display Panel
TopCare® health
COMPARE TO VICKS® NYQUIL® SEVERE HONEY FLAVOR ACTIVE INGREDIENTS
MAXIMUM STRENGTH RELIEF
Nite Time Cold & Flu
SEVERE
PAIN RELIEVER/FEVER REDUCER – ACETAMINOPHEN
NASAL DECONGESTANT – PHENYLEPHRINE HCl
COUGH SUPPRESSANT – DEXTROMETHORPHAN HBr
ANTIHISTAMINE – DOXYLAMINE SUCCINATE
• Headache, Fever, Sore Throat, Minor Aches & Pains
• Nasal/Sinus Congestion & Sinus Pressure
• Sneezing, Runny Nose
• Cough
12 FL OZ (355 mL)
HONEY FLAVOR
-
INGREDIENTS AND APPEARANCE
TOPCARE NITE TIME COLD AND FLU
acetaminophen, dextromethorphan hydrobromide, doxylamine succinate, phenylephrine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76162-202 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg in 15 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 15 mL DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 6.25 mg in 15 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg in 15 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76162-202-40 355 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/03/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/03/2023 Labeler - Topco Associates LLC (006935977)