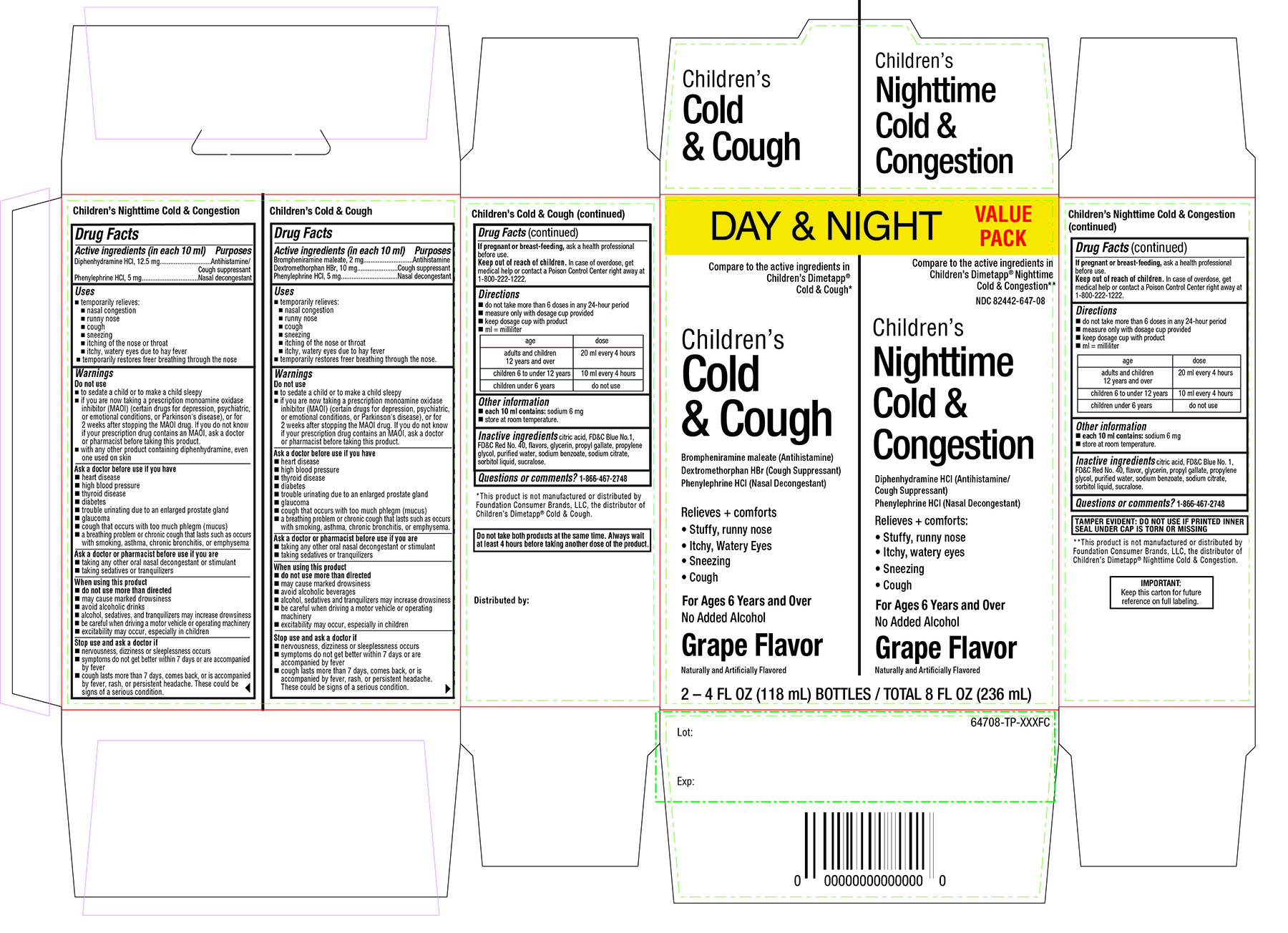
Label: DAYTIME COLD AND COUGH AND NIGHTTIME COLD AND CONGESTION CHILDRENS- brompheniramine maleate, dextromethorphan hbr, phenylephrine hcl, diphenhydramine hcl, phenylephrine hcl kit
- NDC Code(s): 82442-406-04, 82442-607-04, 82442-647-08
- Packager: TARGET Corporation
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated July 4, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients for Nighttime (in each 10 mL)
- Active ingredients for Daytime (in each 10 mL)
- Purpose for Nighttime
- Purpose for Daytime
-
Uses
Nighttime
- temporarily relieves
- nasal congestion
- cough
- runny nose
- sneezing
- itchy, watery eyes due to hay fever
- itching of the nose or throat
- temporarily restores freer breathing through the nose
Daytime
- temporarily relieves
- nasal congestion
- runny nose
- cough
- sneezing
- itching of the nose or throat
- itchy, watery eyes
- temporarily restores freer breathing through the nose
-
Warnings
Do not use
Nighttime
- to sedate a child or to make a child sleepy.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product
- with any other product containing diphenhydramine, even one used on skin.
Daytime
- to sedate a child or to make sleepy
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI)(certain drugs for depression, Psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
Nighttime
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- trouble urinating due to an enlarged prostate gland
- cough that occurs with too much phlegm (mucus)
- a breathing problem or chronic cough that last as occurs with smoking, asthma, chronic bronchitis or emphysema
Daytime
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- trouble urinating due to an enlarged gland
- cough that occurs with too much phlegm (mucus)
- a breathing problem or persistent or chronic cough that lasts such as occurs with smoking, asthma, chronic bronchitis or emphysema
Ask a doctor or pharmacist before use if you are
Nighttime
- taking any other oral nasal decongestant or stimulant
- taking sedative or tranquilizers
Daytime
- taking any other oral nasal decongestant or stimulant
- taking sedative or tranquilizers
When using these products
Nighttime
- do not use more than directed
- may cause marked drowsiness
- avoid alcoholic drinks
- alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Daytime
- do not use more than directed
- may cause marked drowsiness
- avoid alcoholic drinks
- alcohol, sedative and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Stop use and ask a doctor if
Nighttime
- nervousness, dizziness or sleeplessness occurs
- symptoms do not get better within 7 days or accompanied by fever
- cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition
Daytime
- nervousness, dizziness or sleeplessness occur
- symptoms do not improve within 7 days or are accompanied by fever
- cough lasts more than 7 days, comes back or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition
-
Directions
Nighttime
- do not take more than 6 doses in any 24 hours period
- measure only with dosing cup provided.
- keep dosing cup with product
- mL = milliliter
Age
Dose
adults and children 12 years and over
20 mL every 4 hours
children 6 to under 12 years
10 mL every 4 hours
children under 6 years
do not use
Daytime
- do not take more than 6 doses in any 24 hours period
- measure only with dosing cup provided.
- keep dosing cup with product
- mL = milliliter
Age
Dose
adults and children12 years and over
20 mL every 4 hours
children 6 to under 11 years
10 mL every 4 hours
Children under 6 years
do not use
- Other information
-
Inactive ingredients
Nighttime
citric acid, FD&C Blue #1, FD&C red #40, Flavor, glycerin, propyl gallate, propylene glycol, purified water, sodium benzoate, sodium citrate, sorbitol liquid, sucralose
Daytime
citric acid, FD&C Blue #1, FD&C red #40, Flavor, glycerin, propyl gallate, propylene glycol, purified water, sodium benzoate, sodium citrate, sorbitol liquid, sucralose
- Questions or comments?
-
Principal Display Panel
Compare to active ingredients in Children's Dimetapp® Nighttime Cold & Congestion**
NDC 82442-647-08
Children's Nighttime
Cold & Congestion
Diphenhydramine HCl (Antihistamine-Cough Suppressant)
Phenylephrine HCl (Nasal Decongestant)
Relieves + comforts:
- stuffy nose, runny nose
- sneezing
- itchy, watery eyes
- cough
For Ages 6 Years and Over
No Added Alcohol
Grape Flavor
IMPORTANT: Keep this carton for future reference on full labeling
TAMPER EVIDENT: DO NOT USE IF PRINTED INNER SEAL UNDER CAP IS TORN OR MISSING.
**This product is not manufactured or distributed by Foundation Consumer Brands, LLC, the distributor of Children's Dimetapp® Nighttime Cold & Congestion.
DAYTIME
Compare to active ingredients in Children's Dimetapp® Cold & Cough*
Children's
Cold & Cough
Brompheniramine Maleate (Antihistamine)
Dextromethorphan HBr (Cough Suppressant)
Phenylephrine HCI (Nasal Decongestant)
Relieves + comfort
- stuffy nose, runny nose
- cough
- itchy, watery eyes
- sneezing
For Ages 6 Years and Over
No Added Alcohol
Grape Flavor
Naturally and artificially Flavored
2 – 4 FL OZ (118 mL) BOTTLES / TOTAL 8 FL OZ (236 mL)
*This product is not manufactured or distributed by Foundation Consumer Brands, LLC, the distributor of Children's Dimetapp® Cold & cough
Distributed by
- Product Label
-
INGREDIENTS AND APPEARANCE
DAYTIME COLD AND COUGH AND NIGHTTIME COLD AND CONGESTION CHILDRENS
brompheniramine maleate, dextromethorphan hbr, phenylephrine hcl, diphenhydramine hcl, phenylephrine hcl kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82442-647 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82442-647-08 1 in 1 KIT; Type 0: Not a Combination Product 06/17/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 118 mL Part 2 1 BOTTLE, PLASTIC 118 mL Part 1 of 2 TGT CHILDRENS NIGHTTIME COLD AND CONGESTION
diphenhydramine hcl, phenylephrine hcl liquidProduct Information Item Code (Source) NDC:82442-406 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 10 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg in 10 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) PROPYL GALLATE (UNII: 8D4SNN7V92) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82442-406-04 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/17/2024 Part 2 of 2 TGT CHILDRENS COLD AND COUGH
brompheniramine maleate, dextromethorphan hbr, phenylephrine hcl liquidProduct Information Item Code (Source) NDC:82442-607 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BROMPHENIRAMINE MALEATE (UNII: IXA7C9ZN03) (BROMPHENIRAMINE - UNII:H57G17P2FN) BROMPHENIRAMINE MALEATE 2 mg in 10 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 10 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg in 10 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) PROPYL GALLATE (UNII: 8D4SNN7V92) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82442-607-04 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/17/2024 Labeler - TARGET Corporation (006961700)