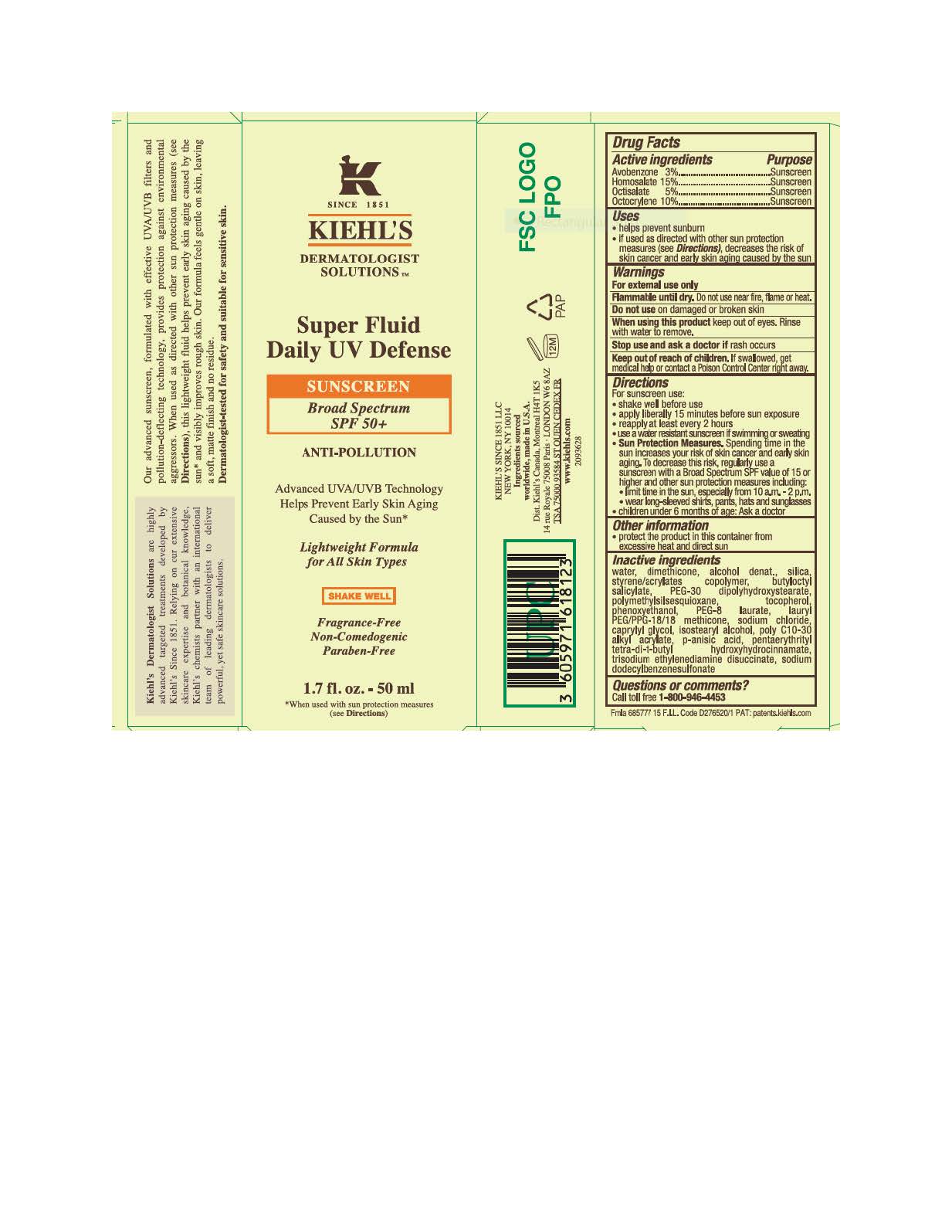
Label: KIEHLS SINCE 1851 DERMATOLOGIST SOLUTIONS SUPER FLUID DAILY UV DEFENSE BROAD SPECTRUM SPF 50 PLUS SUNSCREEN ANTIPOLLUTION LIGHTWEIGHT FORMULA- avobenzone, homosalate, octisalate, and octocrylene lotion
- NDC Code(s): 49967-812-01, 49967-812-02, 49967-812-03
- Packager: L'Oreal USA Products Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 30, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- shake well before use
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, dimethicone, alcohol denat., silica, styrene/acrylates copolymer, butyloctyl salicylate, PEG-30 dipolyhydroxystearate, polymethylsilsesquioxane, tocopherol, phenoxyethanol, PEG-8 laurate, lauryl PEG/PPG-18/18 methicone, sodium chloride, caprylyl glycol, isostearyl alcohol, poly c10-30 alkyl acrylate, p-anisic acid, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, trisodium ethylenediamine disuccinate, sodium dodecylbenzenesulfonate
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KIEHLS SINCE 1851 DERMATOLOGIST SOLUTIONS SUPER FLUID DAILY UV DEFENSE BROAD SPECTRUM SPF 50 PLUS SUNSCREEN ANTIPOLLUTION LIGHTWEIGHT FORMULA
avobenzone, homosalate, octisalate, and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-812 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 150 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ALCOHOL (UNII: 3K9958V90M) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PEG-30 DIPOLYHYDROXYSTEARATE (UNII: 9713Q0S7FO) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-8 LAURATE (UNII: 762O8IWA10) LAURYL PEG/PPG-18/18 METHICONE (UNII: ZJ5S27D9NX) SODIUM CHLORIDE (UNII: 451W47IQ8X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) P-ANISIC ACID (UNII: 4SB6Y7DMM3) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) SODIUM DODECYLBENZENESULFONATE (UNII: 554127163Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-812-01 1 in 1 CARTON 10/01/2022 1 125 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:49967-812-02 1 in 1 CARTON 10/01/2022 2 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:49967-812-03 125 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 Labeler - L'Oreal USA Products Inc. (002136794) Establishment Name Address ID/FEI Business Operations Dimensional Merchandising Inc. 076693183 manufacture(49967-812) , pack(49967-812)